Nov 25, 2020
Moderna soars to record ahead of final COVID vaccine analysis
, Bloomberg News
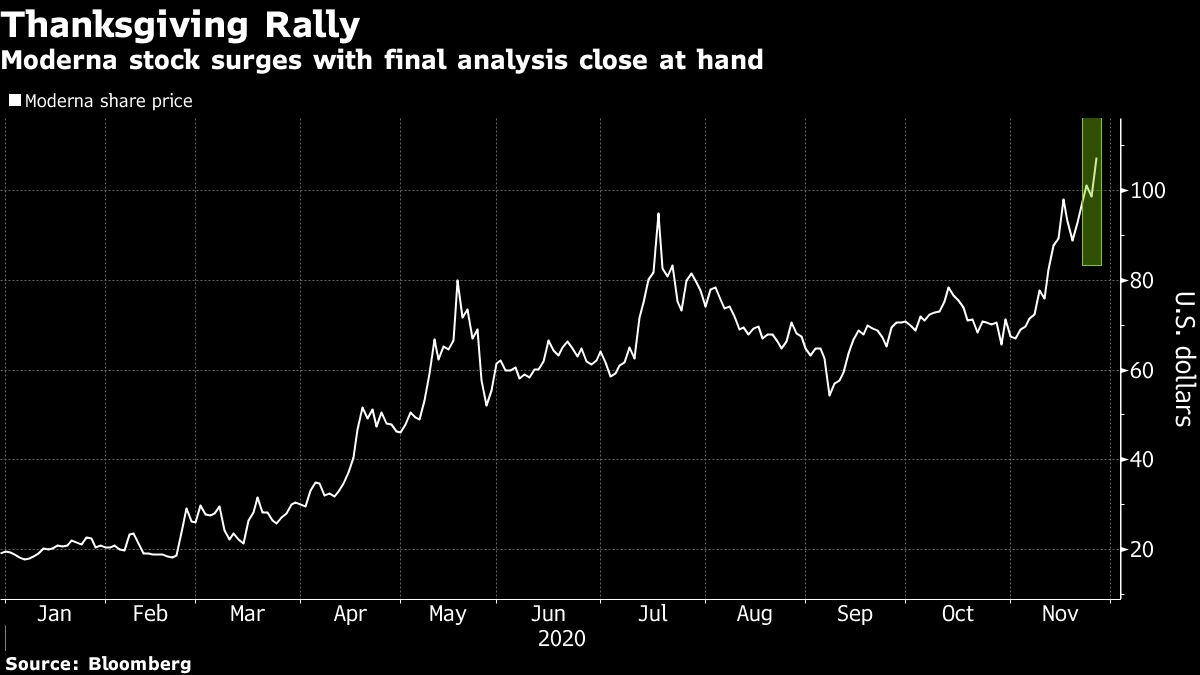
Moderna Inc. climbed as much as 10 per cent hitting a record high on Wednesday while investors await the final analysis of its COVID-19 shots.
That final look at the data is expected within days after 151 volunteers in the 30,000 person study develop symptoms of the virus. It would be the final step before the biotech company files for an emergency use authorization. Moderna is poised to be the second company to do so in the U.S. after Pfizer Inc. was the first to file with U.S. regulators on Friday. Pfizer and German partner BioNTech SE’s shot will face a Food and Drug Administration panel on Dec. 10.
Moderna’s shot was found to be about 94.5 per cent effective in a preliminary assessment, and Pfizer’s vaccinations looked similarly effective in a final analysis. “Look for similar efficacy to be maintained” in Moderna’s last look, Goldman Sachs analyst Salveen Richter wrote in a note to clients. Moderna’s stock price has climbed more than fivefold this year.