Nov 15, 2018
Age-Old, Sexually Transmitted Disease Making a Comeback
, Bloomberg News
(Bloomberg) -- New Zealand managed to quell an infectious child-killer with the help of a new type of vaccine. A decade later, scientists in the South Pacific nation found it may be critical combating an age-old, sexually transmitted infection that’s making a comeback: gonorrhea.
That’s spurring optimism that the fast-spreading disease could be slowed using a vaccine already on the market to prevent its bacterial cousin -- a strain of the so-called meningococcal bacterium notorious for causing potentially deadly meningitis epidemics in college dorms, such as the recent ones on campuses in San Diego and Massachusetts. While gonorrhea isn’t life-threatening, it’s now on the verge of becoming unstoppable due to antibiotic resistance.
Doctors are also reporting that strains of gonorrhea are behaving like related infections that persist in the throat, where the germs can spread surreptitiously via kissing. That’s adding to the urgency of finding new ways to stop the scourge, which has widened its hold on minority groups, including gay men, to the broader community.
Cases jumped 19 percent in the U.S. last year, with similar trends noted around the world. While no immunization against gonorrhea exists, studies show that a licensed vaccine made by GlaxoSmithKline Plc may offer at least partial protection.
‘Lots of Questions’
“Next to the development of a new drug or something like that, there is nothing,” said Helen Petousis-Harris, a vaccinologist at the University of Auckland, who worked with colleagues to show, for the first time, that a custom-made meningococcal B shot was associated with protection against the STI known colloquially as the Clap. “This has opened up a whole program of work that needs to be done with lots of questions to be answered.”
The shot that New Zealand used to immunize 1.1 million people from 2004 to 2006 in people 6 months to 20 years of age was different to typical meningococcal vaccines. It was aimed at stimulating antibodies against an additional feature common to both germs called outer membrane vesicles. Scientists speculate this may be an important source of cross protection.
No one knows yet how that cross protection might work or for how long. Answering those questions is key to determining whether vaccines targeting these outer membrane vesicles, like Glaxo’s Bexsero, might help mitigate the spread of gonorrhea.
‘Unpacking the Magic’
“It’s basically unpacking the magic of finding out what it is,” Petousis-Harris said over the phone from Auckland.
Her paper in the Lancet medical journal last year estimated vaccination against meningococcal B resulted in 31 percent effectiveness against gonorrhea. That invigorated interest in the field, according to researchers at the National Institute of Allergy and Infectious Diseases and the Uniformed Services University in Maryland.
“While Bexsero may not be the exact answer, we’re a lot closer to an answer for a gonorrhea vaccine than we have been in a long time,” said Leah Vincent, a scientist at the institute, in a telephone interview.
$166 Shot
GlaxoSmithKline hasn’t yet made a decision on whether to target gonorrhea with Bexsero, which is forecast to generate $780 million in sales this year purely as a meningococcal B vaccine. The product has a retail price in the U.S. of about $166 per dose.
The drugmaker is reviewing the results of independent studies and talking with health authorities and outside researchers about potentially evaluating Bexsero as a way of preventing gonorrhea, the London-based company said in an emailed response to questions. Meantime, it’s advancing development of the antibiotic gepotidacin to treat the disease after completing two mid-stage studies.
“GSK is aware of the increasing public health need for prevention of gonorrhea, particularly in light of antimicrobial resistance, and is exploring ways to contribute our expertise to this important issue,” the company said.
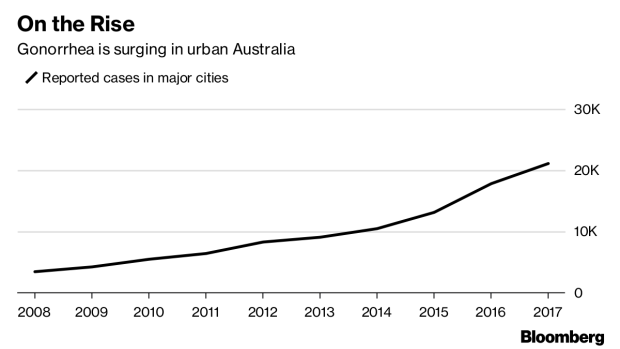
Gonorrhea cases have jumped sixfold in major Australian cities over the past decade, and 78 million new infections occur worldwide each year. The standard two-drug regimen costs $21.66 per treatment in low and middle-income countries, Vincent and Ann Jerse, from the Uniformed Services University, wrote in a paper published in April.
Establishing a patient trial with a two-dose Bexsero regimen targeting American adolescents could prevent almost 84,000 infections and save almost $70 million in total costs, they said.
The sexually transmitted bacteria develops antibiotic resistance rapidly compared with other organisms, Jerse said over the phone. “Even though there are drugs in the pipeline, it’s just going to be a short-term solution. That’s why a vaccine is really important, and the time is now.”
Gonorrhea uses a specific protein to defeat the body’s first line of defense, Jerse and colleagues at the Oregon State University’s College of Pharmacy said in July, opening up a new pathway to develop both novel antibiotics and vaccines.
Infertility, Blindness
Gonorrhea, an ancient disease recognized in the second century by the Greek physician Galen, often causes no symptoms, especially in the throat, enabling it to spread unnoticed. Left untreated, the disease can cause a raft of complications from infertility and ectopic pregnancies, to premature births and blindness.
Cases of gonorrhea for which no standard therapy currently exists have been detected in Australia and the U.K., the House of Commons in London said in a report last month. Health authorities have warned of more extreme cases to come.
“Like many diseases, we forget how bad they were,” said Kate Seib, who helped develop Bexsero in Siena, Italy, for Novartis AG, which sold the vaccine to Glaxo in 2015. “But it doesn’t kill anyone, so people still aren’t worried.”
No study has yet proven that Bexsero can prevent gonorrhea, Seib said. Findings in New Zealand, Cuba and Quebec that show a link between vaccination and a subsequent reduction in gonorrhea are “very promising, but just an observation,” she said.
A quick way to discern whether a vaccine could reduce the risk of gonorrhea would be to test Bexsero in a randomized controlled trial among thousands of gay and bisexual men taking a pill to prevent HIV, Seib said. So called pre-exposure prophylaxis, or PrEP, has helped drive down AIDS-causing infections, but is associated with an annual risk of gonorrhea reaching 40 percent.
If such a study was successful, the results would need to be validated in a larger, general population, which would be “exceptionally expensive” to conduct, said Charlene Kahler, head of infection and immunity at the University of Western Australia’s School of Biomedical Sciences in Perth.
The bacterial causes of meningococcal disease and gonorrhea share 80-90 percent of their DNA, and results of studies using animal models back the use of Bexsero for controlling gonorrhea also. Still, measuring success is challenging since there is no known way to gauge protection against gonorrhea in humans, Kahler said in a telephone interview.
Even a modest level of effect from a vaccine could stem the “almost exponential” increase in gonorrhea worldwide and slow the evolution of drug-resistant cases, said Christopher K. Fairley, director of the Melbourne Sexual Health Centre.
“We have to stop expecting that saying to people ‘reduce the number of partners and use condoms’ will work because it’s not working,” said Fairley, who is also a professor of public health at Melbourne’s Monash University, in an interview. “We have to face up to that reality. We have to start exploring other methods.”
--With assistance from Jason Gale.
To contact the reporter on this story: James Paton in London at jpaton4@bloomberg.net
To contact the editors responsible for this story: Eric Pfanner at epfanner1@bloomberg.net, ;Brian Bremner at bbremner@bloomberg.net, Jason Gale
©2018 Bloomberg L.P.