Aug 18, 2022
AstraZeneca Hits Another Covid Hurdle in Slow Evusheld Rollout
, Bloomberg News
(Bloomberg) -- Well over a year after the rollout of Covid-19 vaccines allowed most people to return to life as usual, Mark Oakley is still hiding away in a summer house in his garden, avoiding contact with even his closest family members.
He’s had five vaccine doses, but the protective effect has been suppressed by medication he’s taking to tame a lung disorder. He thinks his predicament could be helped with Evusheld, an AstraZeneca Plc drug that protects vulnerable people from Covid by injecting them with two antibodies, but he can’t get hold of it.
“Everywhere I’ve looked has drawn a blank,” Oakley, 52, said by phone from his home in Shoreham-by-Sea on the south coast of the UK.
The therapy, which proved in clinical trials to be effective in preventing Covid-19, is helping bring AstraZeneca back to the forefront of the fight against the virus after concerns over blood clots sapped demand for its vaccine last year. But while sales are rising, uptake has been more sluggish than expected with the UK questioning how well the drug works against new variant and some patients worldwide reporting difficulties getting it.
About half a million immunocompromised people in the UK are waiting for the drug, but the government shelved plans to buy it earlier this month citing lack of evidence over how well it works. The US has ordered 1.7 million doses, but less than half a million had been administered as of the start of August.
“The distribution of Evusheld until now hasn’t been like the distribution of a traditional medicine,” AstraZeneca CEO Pascal Soriot said at a briefing with reporters in London in late July. “People didn’t know about it. Doctors didn’t know about it. When they knew about it, they didn’t know where to find it.”
A major sticking point in the UK is that the clinical trials were carried out before the arrival of the omicron variant, now the dominant strain in most countries. Multiple studies indicate that the drug still works against the highly-contagious omicron but may be less effective than against previous strains. Doses may need to be increased to improve effectiveness.
A UK Department of Health spokesperson said the government won’t procure Evusheld at the moment, citing insufficient data on its ability to offer durable protection against omicron. The drug is due to undergo an evaluation to check whether any potential benefits are worth the money the National Health Service would have to pay for it. The US, which granted the first approval for Evusheld, doubled its recommended dosage in February to ensure protection against omicron and then later advised repeat dosing every six months.
Vaccine Race
AstraZeneca is banking on Evusheld’s success to narrow the gap with US rival Pfizer Inc. in the race to fight Covid. Pfizer’s mRNA vaccine has become the best-selling medicine of all time, generating nearly $37 billion in sales in its first year of rollout. This year, it expects another $32 billion from the vaccine and another $22 billion from Paxlovid, an oral antiviral that prevents a Covid-19 infection worsening.
In contrast, AstraZeneca only brought in $4 billion from its vaccine, as the drugmaker honored a promise to sell the shot at cost. While the shot is estimated to have saved more lives globally than any rival vaccine, concern over extremely rare but deadly blood clots and data showing the mRNA vaccine’s superiority as a booster have led to dwindling use.
Read More: Astra’s Covid Vaccine Saved Over Six Million Lives in First Year
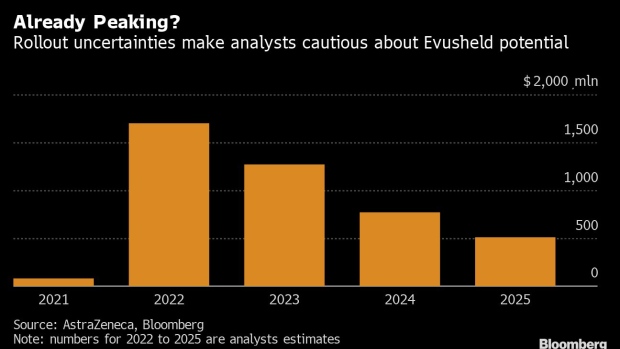
The company cited strong Evusheld sales momentum in the first half as a reason to raise its 2022 revenue growth to more than 20%, but analysts forecast sales will drop next year after peaking at $1.7 billion in 2022.
“We are being conservative in terms of anything with Covid at the moment,” said Susie Jana, a health-care analyst at Shore Capital Group Ltd in London. “It’s better to wait and see rather than forecasting huge amounts of growth.”
China Potential
One place where the drug might gain traction is China, AstraZeneca’s second-biggest market after the US and home to tens of millions of people with cancer and other severe diseases. The company recently struck a deal with Wuxi Biologics to manufacture Evusheld locally, but there’s no guarantee it will get regulatory approval.
About 10% of China’s 1.4 billion-strong population is not yet fully vaccinated because doctors and vaccine centers have been cautious about giving home-grown shots for fear of severe side effects. The shot developed by Pfizer and German partner BioNTech SE remains in regulatory limbo and is not approved for use.
In the UK, charities and advocacy groups have been making repeated pleas to lawmakers to roll out the drug. A joint letter by more than 120 clinicians across the country urged Health Minister Steve Barclay to prioritize making Evusheld available.
Oakley, who hasn’t been to a grocery shop in two and a half years, is optimistic Evusheld will eventually become available so that he can return to his job as a landscaper and hug his three children.
“I remain hopeful,” he said. “I think it’s going to be a case of when we get it rather than if.”
(Updates to add details of omicron study in seventh paragraph.)
©2022 Bloomberg L.P.


