Dec 30, 2021
AstraZeneca-Oxford COVID vaccine approval ramps up U.K. vaccinations
, Bloomberg News
AstraZeneca-Oxford vaccine gets first approval in U.K.
AstraZeneca Plc and the University of Oxford’s COVID-19 vaccine won U.K. clearance, marking the first approval worldwide for a shot that will be key to mass immunizations despite continuing questions over its efficacy.
The vaccine will be prioritized for the country’s most vulnerable groups, with shots starting Monday, according to the government. It’s the second coronavirus injection to be cleared for emergency use in the U.K., after one from Pfizer Inc. and BioNTech SE was authorized in early December.
The move will help the U.K. ramp up vaccinations as a surge in coronavirus infections that’s fueled by a new strain puts growing pressure on hospitals. Prime Minister Boris Johnson told members of Parliament that the approval offers “hope to millions in this country and around the world.”
Britain has launched one of the western world’s swiftest vaccination programs, with at least 800,000 people given the Pfizer shot, the U.K. regulator said Wednesday. It raced ahead of the U.S., which is immunizing an average of 200,000 people a day and has used just a small percentage of available doses in many states.
Despite the need for more vaccines, the U.K. regulator faces questions over its speedy approval of the AstraZeneca shot. The decision “is likely to prove controversial,” Bloomberg Intelligence analyst Sam Fazeli said in a note.
Clinical trials showed different levels of effectiveness for two dosing regimens, with the one that performed better resulting from a mistake. The Medicines and Healthcare Products Regulatory Agency prompted new concerns with its decision to approve a dosage involving two full shots -- the system that showed weaker results in the trial -- as much as 12 weeks apart.
A government advisory group said the priority should be to vaccinate large numbers as quickly as possible rather than completing the regimen right away. Two full shots will be used because there’s incomplete data on the other dosage, involving a half shot followed by a full booster, officials said. Neither dosage, however, was as effective as the Pfizer-BioNTech vaccine or another one, from Moderna Inc.
A panel of scientists behind the U.K. approval said Wednesday that the higher efficacy seen from the regimen involving a half dose could be attributed to the gap between doses in that group rather than the dosing level, which is why they settled on the standard dose. Dose intervals in the clinical trials ranged from four to 26 weeks.
The dosing regimen was chosen because the trial results were strongest with a gap of four to 12 weeks between shots, Munir Pirmohamed, one of the scientists on the committee, said at a briefing. People must wait until day 22 for a level of partial immunity after the first shot, according to the panel.
The trials also left questions about how well the vaccine worked in people over 65, but the regulator said it concluded the vaccine could be given to this age group and more trial data will be available early next year.
A full public assessment of the vaccine will be published shortly, the MHRA said. June Raine, the medicines regulator’s chief executive, said at a press conference that she had “every confidence” in the product’s safety.
A U.S. trial that aims to evaluate the shot in 40,000 people is ongoing and should clarify some of these questions, with results expected early in 2021.
AstraZeneca shares rose as much as 1.8 per cent in London.
Other Approvals
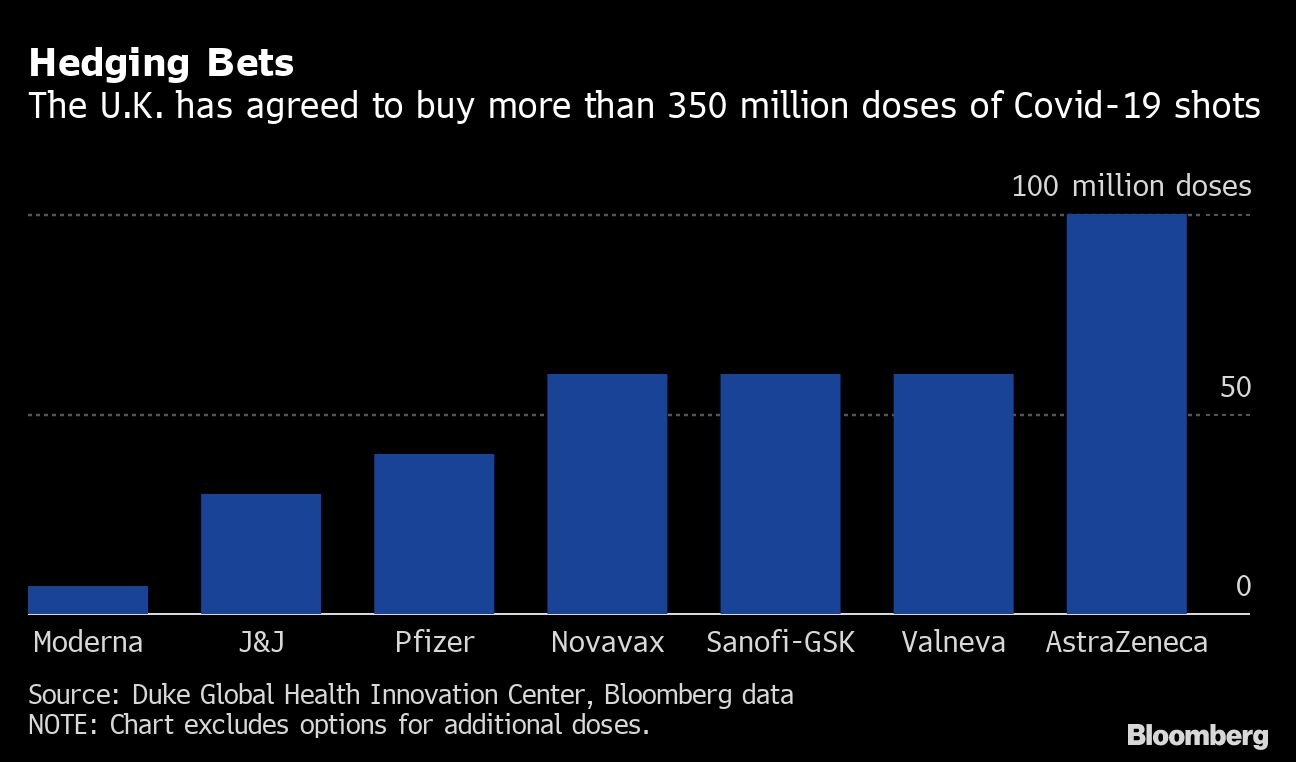
U.K. Health Secretary Matt Hancock said that with the AstraZeneca shot added into the mix, the country will be through the COVID crisis by spring. The vaccine can be deployed swiftly because it’s easier to transport and store than the Pfizer-BioNTech one, requiring only refrigerator temperatures rather than deep freezing. The U.K. has bought 100 million doses.
Also on Wednesday, Argentina approved the shot for emergency use. It may quickly be followed by other countries, including India, though the European Union and the U.S. are not expected to act on the vaccine anytime soon. AstraZeneca said Wednesday it is seeking an emergency-use listing from the World Health Organization so that the shot can be made available to low- and middle-income countries as quickly as possible.
Developing countries have been counting on the shot due to its comparatively easy storage conditions and lower price. The vaccine, which the partners have committed to provide at cost during the pandemic, accounts for more than 40 per cent of supplies going to low- and middle-income countries, based on agreements tracked by London-based research firm Airfinity Ltd.
“This vaccine will be made available to some of the poorest regions of the world at a low cost, helping protect countless people from this awful disease,” Hancock said.