Aug 18, 2022
Bavarian Nordic Near Deal With US Firm to Make Monkeypox Shots
, Bloomberg News
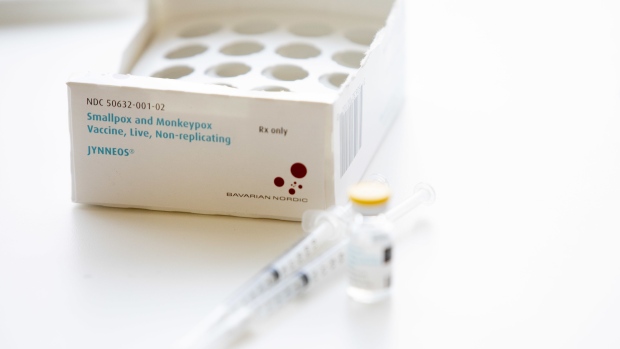
(Bloomberg) -- The Biden administration is aiming to ramp up supplies of scarce monkeypox vaccine by clearing the way for a deal between manufacturer Bavarian Nordic A/S and a Michigan-based company to finish packaging millions of doses in the US.
Grand River Aseptic Manufacturing Inc. reached a deal that would have it ready to fill Bavarian Nordic’s Jynneos vaccine into finished vials within three months, the Danish company and Biden administration officials said in a statement. The technology transfer is already underway, they said. Normally, it would take as long as eight months to get the finishing process up and running, US health officials said.
American depositary receipts of Bavarian Nordic jumped as much as 12% to $18.23 after Bloomberg reported that negotiations were nearly complete. The stock rose as much as 6.6% in Copenhagen early Friday. Neither Bavarian Nordic nor Grand Rapids, Michigan-based Grand River responded to requests for further comment.
The partnership “will significantly increase the capacity to fill and finish government-owned doses -- for the first time in the U.S. -- and allow us to deliver our current and future supply more quickly to locations nationwide,” Bob Fenton, coordinator of the White House national monkeypox response, said in a statement.
Bavarian Nordic has the only vaccine cleared for monkeypox, a viral disease spreading to countries around the world and in the US. Demand for the shot has skyrocketed, leading the company to seek international production partners to help make doses.
“In the short term, we’re trying to respond to monkeypox,” Dawn O’Connell, who leads the Health and Human Services Department’s Administration for Strategic Preparedness and Response, said in an interview. “But in the long term, this is an important addition for us and our preparedness.”
Bavarian Nordic can produce more bulk vaccine than it can fill into vials, Gary Disbrow, director of ASPR’s Biomedical Advanced Research and Development Authority unit, said in an interview. In July, BARDA began introducing the Danish drugmaker to several potential US-based partners and Grand River was ultimately selected.
The priority at Grand River will be finishing an order of 2.5 million Jynneos doses placed in July, US health officials said. The Biden administration will then assess response efforts and whether continued manufacturing capacity is needed. Any extra vaccine doses could be stockpiled or go to other countries that need them, officials said.
Grand River has already faced challenges in obtaining materials and equipment necessary to produce finished vials of Jynneos, according to the people familiar with the matter, who asked not to be named as the details weren’t public.
The Biden administration worked directly with suppliers to rapidly acquire equipment and materials, Disbrow said, such as vials, disposable bags and tubing that have also been needed to make vital Covid vaccines.
“We’re now feeling confident that we’re on track,” he said in an interview. “The suppliers stepped up to the plate.”
Bavarian Nordic is also looking at a potential partnership with a large US pharmaceutical company to increase manufacturing capacity, O’Connell said on a press call Thursday.
Short Supply
When monkeypox cases started cropping up in May, US health officials thought the country’s 2,400 stockpiled shots would be sufficient. During past outbreaks, the virus primarily spread through direct contact with infected animals and was easily contained.
Surprisingly, the outbreak has spread quickly and widely, especially among gay and bisexual men. The virus is mainly transmitted through close, intimate contact, but cases linked to casual contact have been reported.
Health officials began thinking about shoring up domestic manufacturing capacity as monkeypox cases climbed in Europe, suggesting US cases would rise.
Three months later, the US has the highest number of monkeypox cases globally, accounting for almost a third of the world’s infections. Millions of doses are on order, but getting them into arms has been slowed by manufacturing snags, regulatory hurdles and contract negotiations. So far, vaccines have gone primarily to White people, raising equity concerns similar to those seen with Covid shots.
The US has access to bulk vaccine materials equivalent to about 13 million doses and has received about 88% of all Jynneos vaccines delivered since the outbreak started in May; still, many Americans are struggling to find shots. Health officials estimate that up to 1.7 million Americans are at highest risk of getting the disease, far more than the government’s dwindling stockpile of 400,000 doses can inoculate.
Shortages prompted officials to greenlight a vaccination technique that uses just one-fifth of a normal dose to stretch supplies while they remain limited. This strategy could turn the 400,000 doses into some 2.2 million, health officials have said. But state health departments have warned that the technique, which involves injecting the vaccine between layers of skin, requires training and specialized syringes that may delay implementation for weeks.
In a letter to US health officials, Bavarian Chief Executive Officer Paul Chaplin said the US failed to coordinate with the company prior to announcing the dose-sparing strategy. Instead, the CEO said he supports delaying the second of the two-shot regimen, which some states have already begun doing, until more supply is available.
US health officials have defended the decision to stretch supplies via this technique and plan to continue using it even as supply increases.
“We messed up a little bit on the messaging by originally calling it ‘dose-sparing,’” said David Boucher, ASPR’s director of infectious disease preparedness and response. “It’s a strategy that enables us to vaccinate more individuals, but dose-sparing implies a less than optimal dose.” Health officials are now trying to “correct the public messaging,” he said.
How effective the vaccine is at preventing monkeypox in humans -- even at full doses -- remains unclear; most studies of its use against the disease were done in animals, as monkeypox outbreaks in humans are typically rare and small.
As long as FDA and CDC are “recommending that strategy, we will continue to stand by our colleagues,” O’Connell said.
(Updates to add share price reaction in third paragraph.)
©2022 Bloomberg L.P.