Mar 31, 2023
Biotech Stocks Languish After Dismal Quarter of Clinical Results
, Bloomberg News
(Bloomberg) -- Biotechnology stocks have been under pressure for two years as investors pivot away from risky assets, but a more fundamental problem emerged in the first quarter: bad clinical trial results.
Drug developer stocks — which often stage wild swings based on the outcomes of clinical trials of their experimental therapies — have been languishing this year as positive data readouts for middle and late-stage studies dropped to 35% in the quarter, according to Jefferies data as of Wednesday. That’s far below the historical average of about half the studies hitting their targets, and marks the third-worst quarter in the past five years.
“Add in freshly renewed concerns about a lack of access to capital following the SVB situation, along with increased fear over drug pricing, and it’s no surprise that biotech is down,” Jefferies strategist Will Sevush said by email.
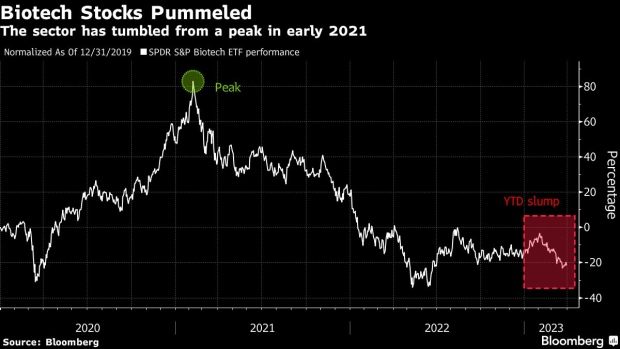
The SPDR S&P Biotech ETF (ticker XBI) — which holds beaten-down stocks including Altimmune Inc. and Bluebird Bio Inc. — has dropped more than 8% since the start of the year, lagging gains seen by the broader market.
The collapse of Silicon Valley Bank, a key financing cog in the life sciences sector, also rattled investors across the space and prompted companies to disclose their exposure to the firm. But unlike industry or macro headwinds that impact competitors alike, the outcome of clinical trials are far more idiosyncratic.
Among stocks that plunged after trial flops was Nektar Therapeutics, which tumbled nearly 50% to a record low in February after a mid-stage study of a potential lupus drug missed its main goal. Earlier this month, Aclaris Therapeutics cratered erasing $380 million in market value in a single day after a trial in a common skin condition disappointed.
Investors and companies’ fortunes could reverse in the coming quarters. Last year’s dismal first half was followed by much stronger readouts in the latter part of the year. Regeneron Pharmaceuticals Inc. and Sanofi reported positive data from a chronic obstructive pulmonary disease trial last week.
FibroGen Inc. has multiple late-stage trials set to readout in the coming months, while data on Cytokinetics Inc.’s closely watched heart drug is expected in the fourth quarter.
--With assistance from Bre Bradham.
©2023 Bloomberg L.P.






