Mar 19, 2023
Here’s a Radical Tactic to Bypass UK Health Logjam: Look Abroad
, Bloomberg News
(Bloomberg) --
Laura Nuttall was a few weeks into her first year studying at King’s College London when she was diagnosed with brain cancer and UK doctors gave her about 12 months to live.
More than four years later, Nuttall has defied expectations and completed her bachelor’s degree after her family searched for alternative treatments and used crowdfunding to pay for therapy outside the National Health Service’s remit, both at home and abroad.
The Nuttalls broadened their quest when they bumped against the constrains of a UK health system choked by lengthy waiting lists, overwhelmed medical staff and a lack of funding.
“The reason that she’s still here is because we’ve looked outside of the box,” Nicola Nuttall, Laura’s mother, said in an interview.
The NHS’s woes don’t just affect patients who can’t find drugs or clinical trials to help boost their survival chances. Millions of people are in limbo, waiting to get treated. Doctors’ and nurses’ frustration has escalated into a series of strikes, and scientists are looking for jobs with better research opportunities elsewhere.
Even some pharma companies now admit they’re opting to conduct clinical research — a crucial juncture for science and for patients, who can gain access to promising medicines before they’re approved — in other countries.
“The clinical-trial environment in the UK is at risk of becoming irreversibly damaged and harming patient access to innovation,” said Laura Steele, one of US drugmaker Eli Lilly & Co.’s top executives in Europe.
France’s Sanofi recently chose another destination for a study that required quick patient recruitment. The plan was to conduct the early-stage trial in the UK, but “delays in that process” prompted a shift to another European country, Jessamy Baird, who heads Sanofi in the UK and Ireland, said in an interview.
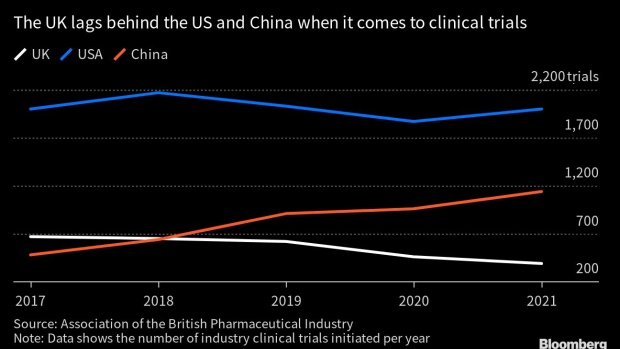
Sanofi estimates it has about 64 studies in the UK that are active or are in planning so far this year, noting a drop of about 26% since 2020. Lilly, known for its cancer and diabetes blockbusters, figures that when it comes to setting up clinical trials, the UK now ranks second from the bottom among the 18 European countries it serves.
Right to Try
Prime Minister Rishi Sunak’s government underscored the emergency last month by announcing a swift review of the landscape for such tests.
For Laura Nuttall’s aggressive form of brain cancer, known as glioblastoma, the UK offers surgery, radiotherapy and chemotherapy with an older drug, temozolomide. The standard protocol is similar elsewhere, but the main difference is the flexibility that patients have to try experimental treatments when their life is at risk.
In the US, eligible patients have a “right to try,” meaning they can request to use medicines that aren’t yet approved under certain conditions. There’s a similar initiative in Germany.
Nuttall didn’t immediately consider going abroad after scans revealed brain tumors following bouts of headaches. She underwent surgery at a hospital near Manchester operated by the NHS, Salford Royal. After the operation, she got radiotherapy and chemotherapy and her family started researching other options to complement the UK protocol.
Crowdfunding Effort
They first found a US company with a London presence that made a form of personalized vaccine out of patients’ own cells but required crowdfunding, because it wasn’t available through the NHS.
In the end, Nuttall didn’t have enough tumor tissue stored to continue down that path, but her family found another treatment avenue: a clinic in Germany offering a mix of immunotherapy that included electro-hyperthermia — a way of heating body tissue to damage cancer cells.
“Obviously we have no guarantees but we’re optimistic that this is the best course of action for her,” Nicola Nuttall wrote on the GoFundMe crowdfunding page.
Laura started going to Cologne for treatment over the summer of 2019, and kept it up through the pandemic. “It’s exciting in a way, because you get to be on the frontier of brain cancer solutions hopefully,” she said by phone. “And obviously it’s helped as well.”
After a second brain surgery in 2021, the family launched another fundraising appeal to help pay for treatments like Merck & Co.’s Keytruda, which isn’t available via the NHS for glioblastoma or covered by their private health insurance. In total, they raised more than £250,000 ($304,325) so far.
The Nuttalls also paid for DNA and RNA analysis of the tumor in Germany. The information was sent to a doctor in Los Angeles who used artificial intelligence to match the tumor’s specific mutations with drugs that might help. But the family got frustrated at how difficult those medicines were to access in the UK.
“You have the information, you have the raw data, but it still can get you absolutely nowhere,” Nicola Nuttall said.
One of the medicines was AstraZeneca Plc’s Lynparza, which the NHS only uses to treat ovarian cancer. The drug is currently being tested for glioblastoma in the UK, but Nuttall can’t join the clinical trials — which combine the pill with radiotherapy — for various reasons, including the requirement for patients to be newly diagnosed with the cancer.
The Nuttalls have yet to find a clinical trial that’s suitable for Laura. The crunch at the NHS is narrowing options since such tests rely on medical staff to refer patients to studies. Sometimes front-line care and research compete for the same resources, according to Jennifer Harris, director of research policy at the Association of the British Pharmaceutical Industry.
“Protecting time for research is increasingly difficult,” Ian Walker, executive director of policy, information and communications at Cancer Research UK, said by email. “If this pattern continues, it means slower progress toward brand new treatments for cancer.”
The number of clinical trials initiated in the UK fell by 41% between 2017 and 2021, according to the ABPI, which found studies are also slower to get started.
The UK has shown it’s able to act swiftly to bring potentially life-saving medical products to the public. Thousands of Brits took an experimental Covid-19 vaccine developed by AstraZeneca and the University of Oxford over the summer of 2020 as part of a large-scale study, and the country was one of the fastest in clearing new vaccines during the pandemic.
Always Growing
More than four years after her diagnosis, Nuttall is still persevering. She’s had four tumor-removal surgeries, several rounds of radiotherapy and almost two years of chemotherapy. Lately her family’s private health insurance has helped her access medicines that aren’t covered by the NHS for brain cancer, such as Roche Holding AG’s Avastin — a drug approved for glioblastoma in countries including the US and Switzerland but not licensed in the UK.
Nuttall takes multiple tablets and supplements every day and is now trying a new form of treatment in Germany, which involves a harmless virus being injected directly into the tumor site via a very long, thin needle.
“It’s a little bit scary because it’s quite an innovative treatment, but unfortunately brain tumors like Laura’s always grow back,” her mother said. “So you have to be one step ahead at all times.”
--With assistance from Tim Loh.
©2023 Bloomberg L.P.






