Nov 30, 2020
Investors take note: Antibe’s revolutionary pain drug is entering Phase 3 trials

- Proprietary hydrogen sulfide drug platform poised to significantly change the way pain and inflammation is treated
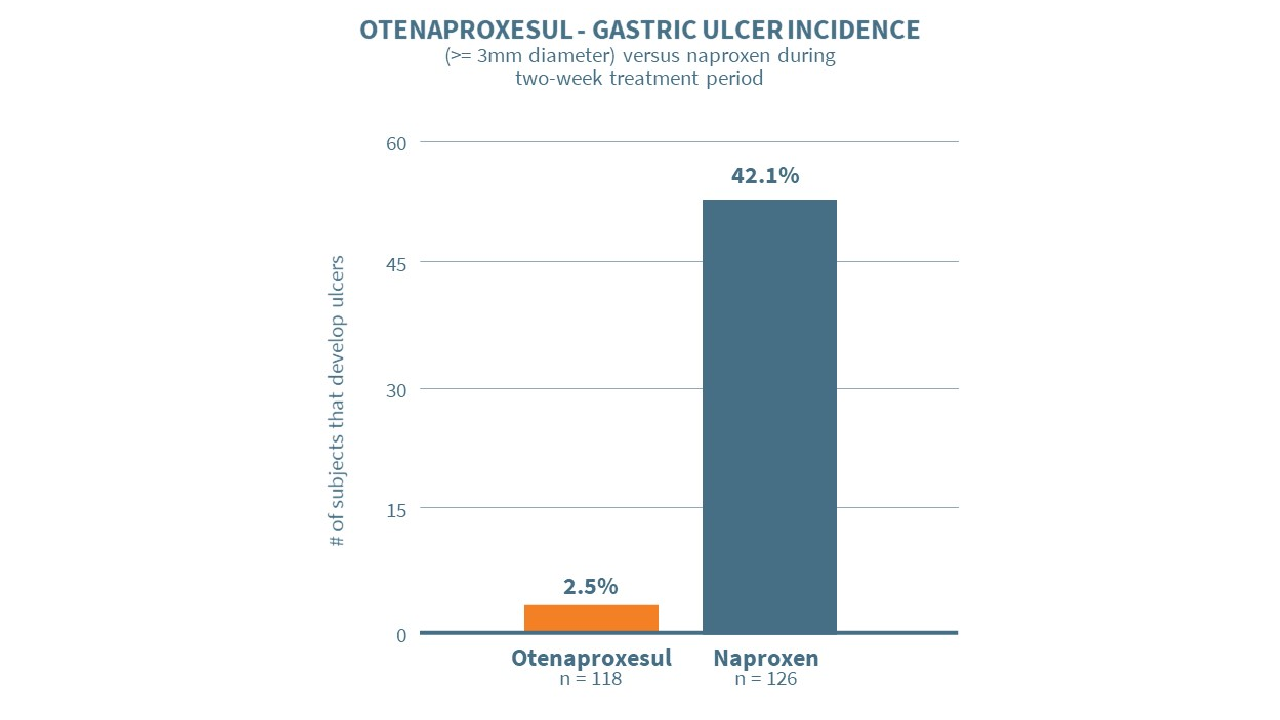
- Lead drug otenaproxesul has shown to dramatically reduce stomach ulcers compared to naproxen in clinical trials
- In June 2020, Antibe reported positive top-line results from its Phase 2B dose ranging efficacy study designed to validate the efficacy of otenaproxesul in reducing pain
Known as pain relievers, brand-name medications like Advil or Aleve have grown in popularity due to their easy-access worldwide availability and recognized efficacy.
These types of medications are known as nonsteroidal anti-inflammatory drugs (NSAIDs), which are taken regularly by approximately 15 per cent of the U.S. population — and among sporadic users, this grows to more than 30 billion doses each year, according to Robert H. Shmerling, MD, Senior Faculty Editor at Harvard Health Publishing in his blog about anti-inflammatory medication.
Although widely available, these NSAIDs do come with risks, if not taken properly. Dr. Shmerling adds that problems that often arise from NSAIDs include digestive and kidney issues, mild allergic reactions or less commonly, severe allergic reactions and liver issues. Cardiovascular considerations are also a concern, although he notes that this risk is dependent on the specific NSAID and the health of the person taking the drug.
Antibe is advancing its novel drug platform
Enter Antibe Therapeutics (TSX: ATE | OTCQB: ATBPF), a clinical-stage biotechnology company currently leveraging its unique hydrogen sulfide platform to develop safer medicines for pain and inflammation.
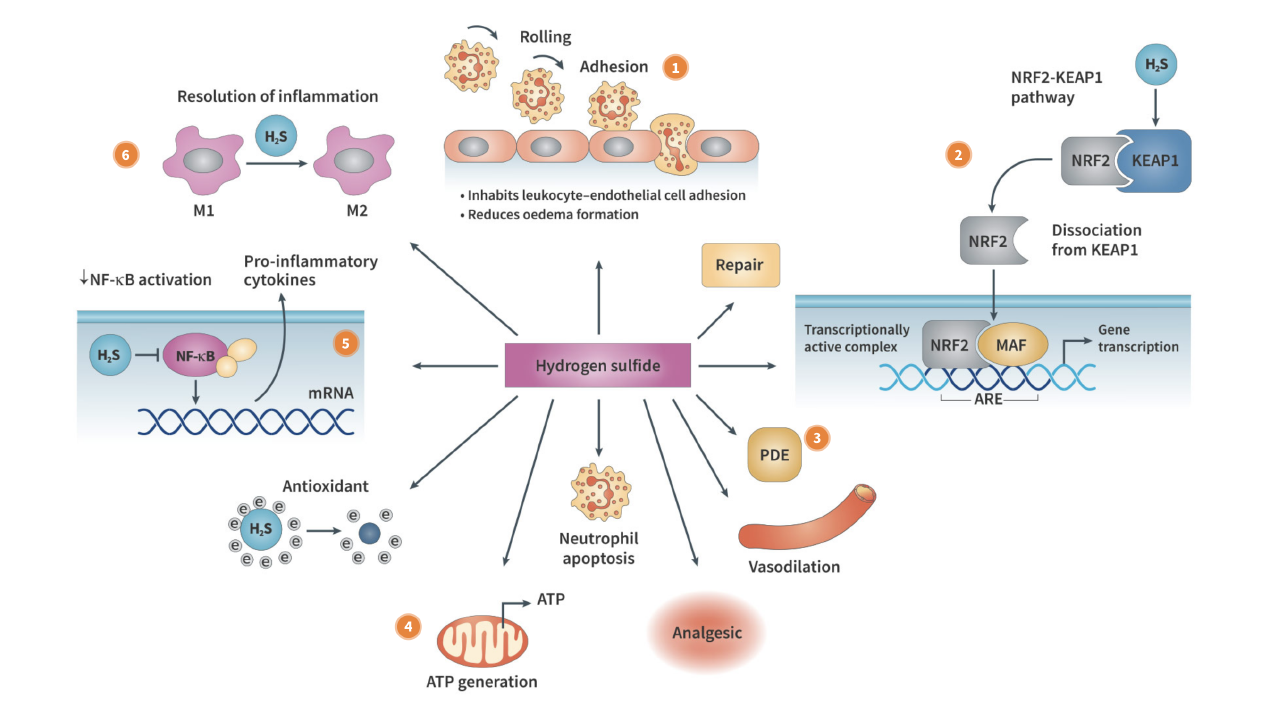
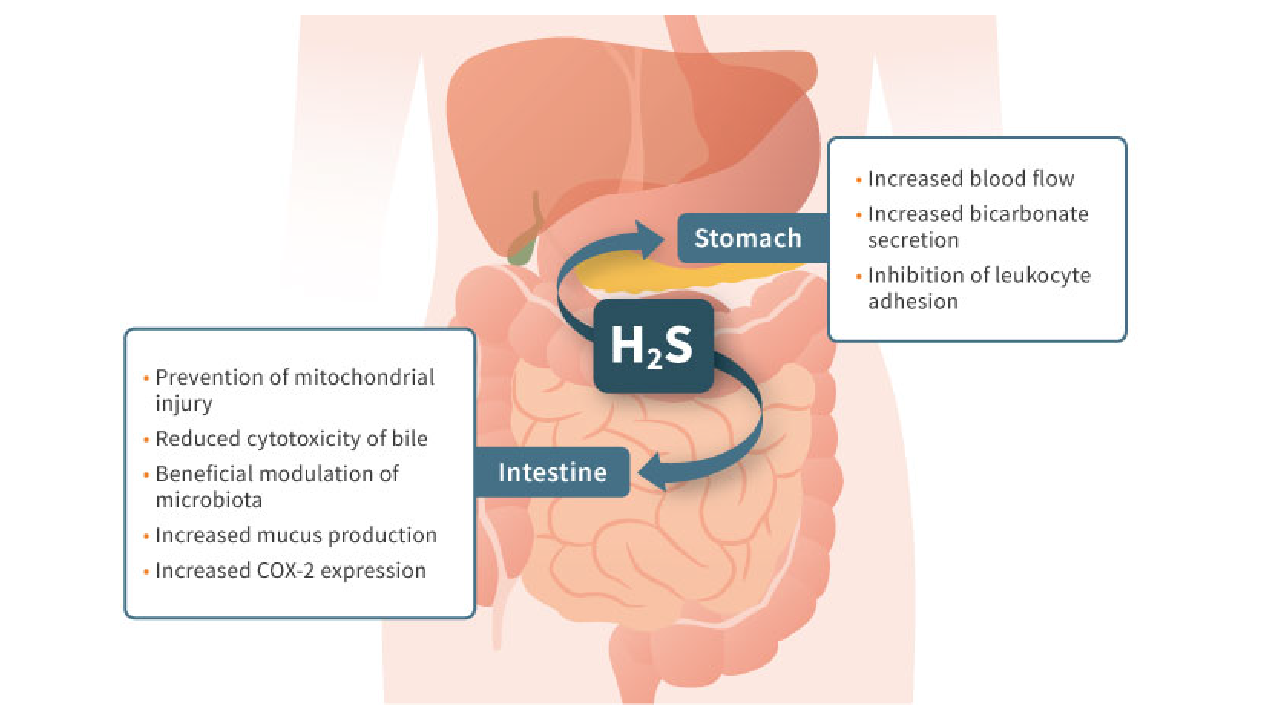
The company is developing a platform of unique drugs designed to prevent serious gastrointestinal damage and bleeding caused by NSAIDs, which are the world’s primary therapy for pain relief.
The company’s lead drug, otenaproxesul (previously known as ATB-346), is a novel anti-inflammatory drug that releases hydrogen sulfide. It has shown unequivocal superiority to naproxen in gastrointestinal safety in a Phase 2B clinical trial. Recently, it demonstrated robust analgesic efficacy against osteoarthritis at a high degree of statistical significance, also in a Phase 2B clinical trial.
Otenaproxesul works to target mild to moderate pain relief that arises from a range of conditions, designed to deliver gastrointestinal safety with non-addictive pain relief. Antibe is also advancing ATB-352 as an alternative to opioids for post-operative pain and ATB-340, a gastrointestinal-safe version of low-dose aspirin.
With clinical results to back up the science and the expertise to advance the company, Antibe is ready to take on the global NSAID market.
A strong management team supported by a global network of advisors
Antibe is led by CEO, Dan Legault, an entrepreneur with extensive experience leading early-stage companies. He has assembled an expert team of advisors and board members, including pharmacologist Dr. Louis Ignarro, a Nobel laureate for his work in demonstrating the signalling properties of nitric oxide, a substance that works synergistically with hydrogen sulfide in the body.
The company’s team also includes John, L. Wallace PhD, MBA, who is the founder as well as Chief Scientific Officer and Director. Dr. Wallace is widely recognized as an expert in gastrointestinal inflammation and in the development of innovative anti-inflammatory medicines.
In 2003, Dr. Wallace and his colleagues identified hydrogen sulfide’s anti-inflammatory properties. Over the succeeding years, their work would demonstrate hydrogen sulfide’s gastrointestinal-protective potential, leading to the design of Antibe’s drug platform.
This year, the company hired Dr. Joseph Stauffer as its Chief Medical Officer. Trained as an anesthesiologist, Dr. Stauffer is a former FDA Medical Review Officer with extensive experience directing medical affairs for pain drug companies including Abbott Laboratories.
Antibe is tapping into a massive market in the healthcare sector
According to a market report from Fortune Business Insights, the NSAIDs market is projected to hit USD$24.35 billion by 2027, growing at a CAGR of 5.8 per cent during the forecasted period.
Tapping into this market, over the summer Antibe revealed plans to ramp up discussions with potential global partners to monetize its drug pipeline for the large pharmaceutical markets. Given the strength of existing clinical results, Legault says the company is “Well-positioned” to deliver high-value partnerships.
The company's Phase 2B efficacy study has a total of 384 patients across 39 clinical sites, in "One of the largest clinical studies ever undertaken in Canada.” In June 2020, the results of the trial validated the best-in-class potential of the drug in one of the world’s largest pharmaceutical categories, setting the stage for global partnering activity.
Company news and milestones
In November 2020, Antibe announced that it had received final approval from the TSX to list its common shares on the Exchange. This graduation from the TSXV to TSX represents a further step in the company reaching the broader investment community while increasing liquidity for its shareholders.

The company also released its Q2 2021 interim financial and operating results.
Highlights:
- Announced robust secondary data from otenaproxesul’s Phase IIB dose-ranging, efficacy study, confirming the drug’s remarkable potency shown in earlier top-line results
- Completed third party studies for the seven largest markets, framing an impressive commercial opportunity for otenaproxesul
- Initiated two animal toxicology studies required for all companies by the U.S. Food and Drug Administration to begin Phase III trials (six additional such studies have commenced in the current quarter)
- Launched pipeline expansion initiatives aimed at developing new intellectual property based on the company’s hydrogen sulfide platform
- Commenced a proprietary naming initiative for otenaproxesul with a leading global branding agency to determine the commercial brand/trademark
- Engaged Stern IR, a premier investor relations agency for healthcare and biotechnology companies worldwide, to support Antibe’s institutional outreach in the U.S.
- Completed the quarter with a cash balance of $22.5 million
Legault notes, “We’ve made tremendous progress this quarter in preparing our lead drug for Phase 3 trials and partnering. We are getting closer to the start of our Phase 3 program, with a total of eight Phase 3-enabling studies now running in parallel.”
“Additionally, the recent commercial data strengthens our position as we engage partners for the large markets. Finally, we are executing our capital markets strategy to increase institutional awareness and liquidity, highlighted by the recent graduation to the TSX.”
He adds, “We look forward to providing a comprehensive corporate update in the coming weeks.”
For investors interested in biotechnology, or for those who are looking to diversify their portfolio, learn more about Antibe Therapeutics by visiting their website here.
For additional information, watch Antibe’s C-Suite interview with the TMX Group below.

Make sure to follow Antibe on social media:
This article was adapted from Proactive Investors and has been updated to reflect the company’s latest information.