Nov 9, 2020
Novavax COVID-19 vaccine gets fast-track tag from FDA
, Bloomberg News
COVID-hurt stocks will be fully recovered within a year if the vaccine goes ahead: Portfolio manager
Novavax Inc.’s experimental COVID-19 vaccine received a fast-track designation from U.S. regulators as the drugmaker prepares to launch a large, late-stage study before the end of the month, the company said on Monday.
The expedited review by the U.S. Food and Drug Administration could help place the company’s candidate among a short-list of frontrunners in the race to bring a vaccine to market.
Some of the most high-profile vaccines are moving closer to approval. Also Monday, Pfizer Inc. and BioNTech SE reported preliminary results showing their vaccine candidate was 90 per cent effective in a late-stage trial. The New York-based drug giant and its German partner could be the first to seek an emergency use authorization in the U.S. The companies received a fast-track designation from U.S. regulators in July.
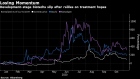
Shares of Novavax were up 6.9 per cent at 10:10 a.m. in New York, building on the stock’s enormous gains so far this year. Since Dec. 31, Novavax has seen its market value increase more than 2,300 per cent .
Novavax has received US$1.6 billion from the U.S. government’s Operation Warp Speed program to speed development and manufacturing of coronavirus vaccines and therapies. The funds will allow the company to conduct advanced human studies of its two-shot regimen, and establish manufacturing to deliver 100 million doses as soon as late 2020.
The Gaithersburg, Maryland-based company launched Phase 2 trials in the U.S. and elsewhere in August. Its vaccine, which consists of synthetic spike proteins grown in armyworm moth cells, lags behind those that launched large, late-stage trials at the outset of the summer.
Novavax expects to begin its final-stage study of its candidate, NVX-CoV2373, in the U.S. and Mexico by the end of November. It had originally expected the 30,000-person trial to start enrolling in mid-October.
An ongoing Phase 3 study from Novavax in the U.K. is expected to be fully enrolled by the end of November, the biotech said in the statement, adding that interim data in the U.K. trial are expected as soon as the outset of 2021.
In August, Novavax reported results from an early trial. Its two-dose regimen when administered concurrently with immune-boosting technology generated antibody responses that were four times higher than those seen in people who had recovered from the disease, it found.