Dec 1, 2020
Pfizer, BioNTech seek clearance to sell COVID vaccine in Europe
, Bloomberg News
Moderna to seek emergency use of COVID vaccine in U.S., EU
Pfizer Inc. and partner BioNTech SE sought regulatory clearance for their Covid-19 vaccine in Europe, putting the shot on track for a potential approval there before the end of the year.
The formal application submitted on Monday caps a rolling review process that started on Oct. 6 and allowed Europe’s drugs regulator to examine data on the vaccine as it emerged. In November, a study of almost 44,000 people showed the shot prevented 95 per cent of symptomatic coronavirus cases. There were no significant safety problems.
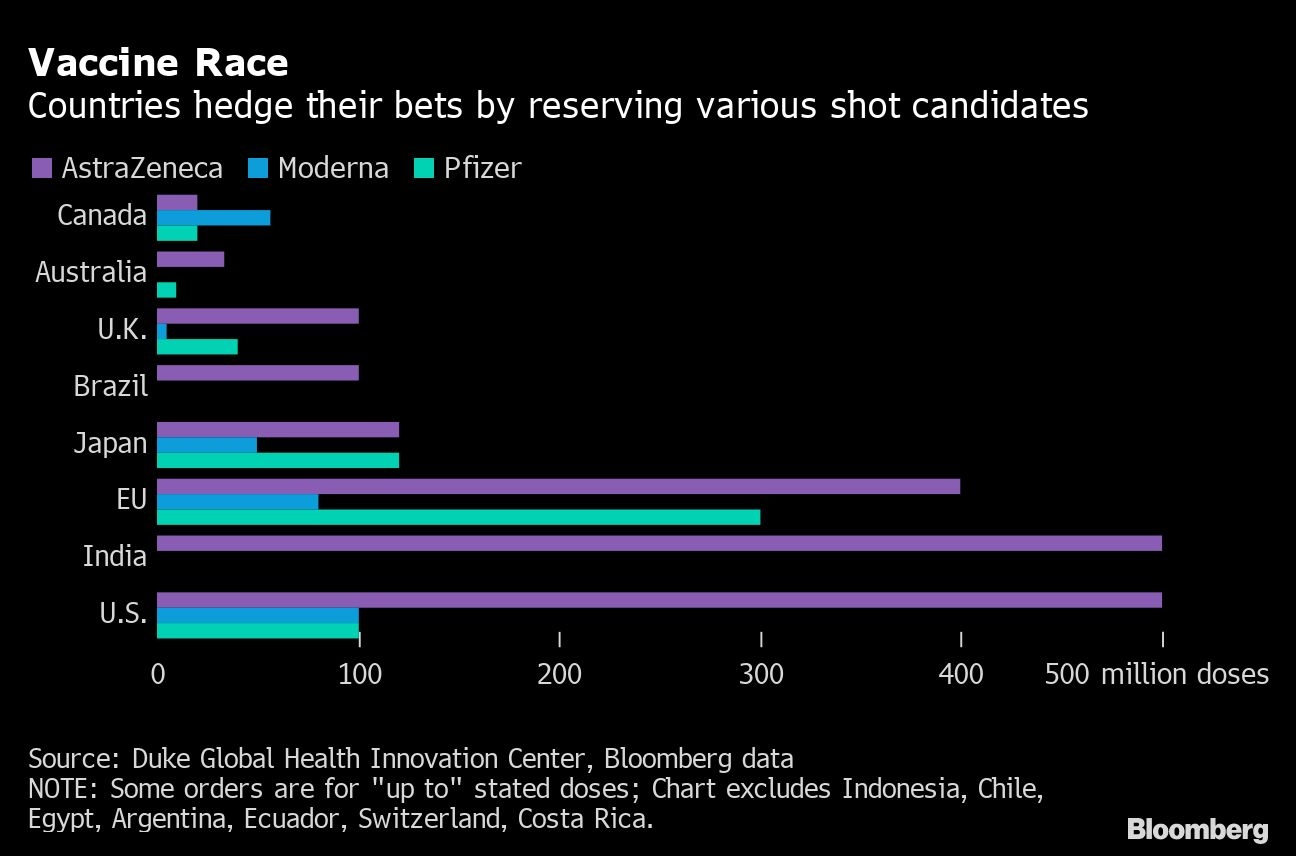
Regulators around the world are racing to review inoculation data, with governments eager to start vaccinating their populations to curb the pandemic. Rival Moderna Inc. requested clearance in the U.S. and Europe on Monday. The U.K. invoked a special rule to allow its regulator to bypass its European Union counterpart and may be the first to sign off on the Pfizer-BioNTech product. The U.S. isn’t far behind, with a Food and Drug Administration panel set to meet on Dec. 10 to discuss the vaccine.
If the European Medicines Agency concludes that the benefits of the Pfizer-BioNTech shot outweigh its risks, it will recommend granting a conditional clearance that could enable the shot to be rolled out in Europe before the end of the year, the companies said in a statement.
Pfizer and BioNTech also started regulatory submissions in other countries including Australia, Canada and Japan, they said. The partners have signed deals to deliver hundreds of millions of doses of the vaccine, including an agreement with the EU for 200 million doses, with an option for an additional 100 million.
A conditional clearance is issued when European authorities want to get a drug to patients quickly without waiting for as comprehensive a data package as would normally be required for standard authorization. It’s valid for one year and can be renewed -- and eventually converted into a standard approval.