Oct 5, 2020
Regeneron gets the 'ultimate validation' after Trump's treatment
, Bloomberg News

Regeneron Pharmaceuticals Inc. climbed the most in more than three months on Monday after U.S. President Donald Trump received the biotech company’s antibody cocktail to treat COVID-19.
President Trump’s treatment was the “ultimate validation” for Regeneron, according to SVB Leerink analyst Geoffrey Porges.
Like Regeneron, Eli Lilly & Co. and AbCellera Biologics Inc. are developing an antibody therapy, not only as a treatment for the virus but also as a preventative. When used as a prophylactic, these products could be considered a passive vaccine as opposed to the active shots most people think of as a vaccine, Bloomberg Intelligence’s Sam Fazeli said last week. AstraZeneca Plc, as well as GlaxoSmithKline Plc and partner Vir Biotechnology Inc. are testing similar therapies.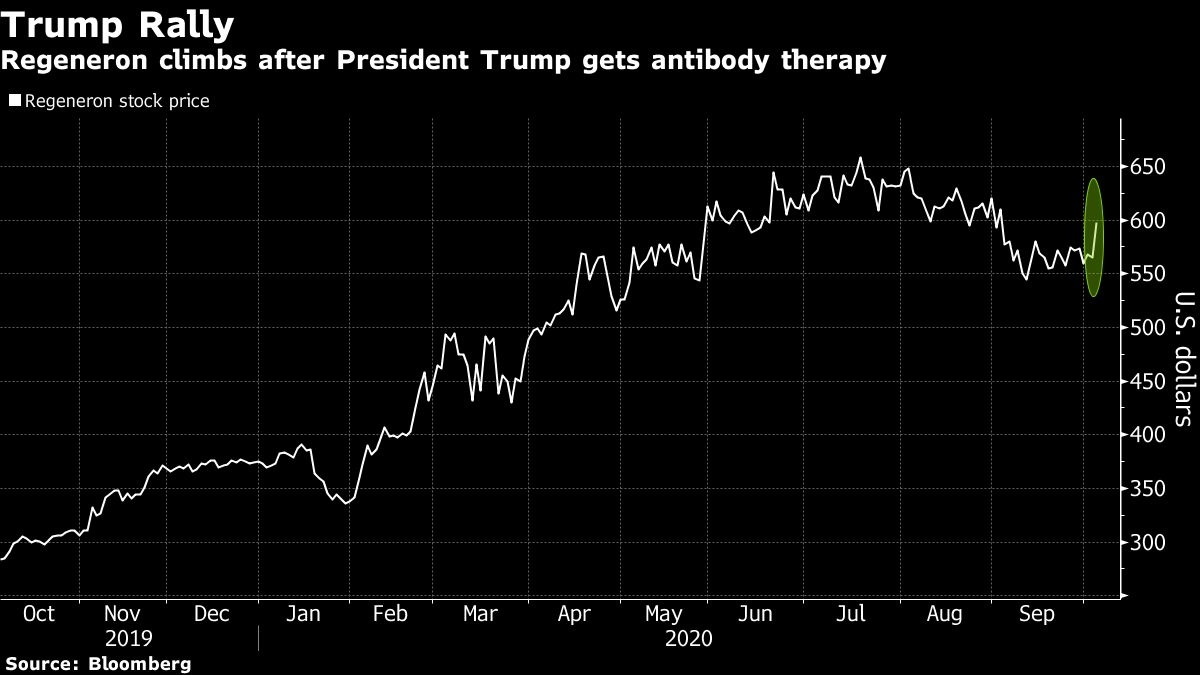
Trump has been promising a vaccine “surprise” before the November election, while the Food and Drug Administration has struggled under the pressure to uphold its scientific measured approach as the administration lobs accusations of stalling at the agency.
Regeneron therapy may get an emergency regulatory authorization in a “matter of days,” according to Porges. “We had an emergency use already in the case of the President. It’s hard to use it in that case and not make it available to the general public,” he said in a phone call.
It was one of several medicines Trump received, along with Gilead Sciences Inc.’s remdesivir and the generic steroid dexamethasone. That’s an unusual step, according to Porges, who has a degree in medicine from the University of Sydney. He said he wasn’t aware of any other COVID-19 patients who had been treated with all three.
A tweet from the president saying he would return to the White House Monday evening after being treated at Walter Reed Medical Center also helped fuel the rally into the end of the trading day. Regeneron shares closed up 7.1 per cent, the biggest one-day gain since June 19. The stock has climbed 61 per cent this year.
I will be leaving the great Walter Reed Medical Center today at 6:30 P.M. Feeling really good! Don’t be afraid of Covid. Don’t let it dominate your life. We have developed, under the Trump Administration, some really great drugs & knowledge. I feel better than I did 20 years ago!
— Donald J. Trump (@realDonaldTrump) October 5, 2020
“Both the company and the White House’s medical staff are likely to have had access to much more information about REGN-COV2 than the company has disclosed publicly so far,” Porges said in a research note. “Given those circumstances, it seems likely that the full dataset for the treatment will be as good or better than the initial 275 patients from one trial disclosed last week.”


