Aug 4, 2020
Singapore Will Get First Claim to Any Successful Arcturus Vaccine
, Bloomberg News
(Bloomberg) -- Singapore sought out U.S. Covid-19 vaccine developer Arcturus Therapeutics Holdings Inc. and is funding its research in order to secure the first doses of any successful final product, in a reflection of the growing urgency in the race for immunization.
San Diego-based Arcturus, in a partnership with Duke-NUS Medical School, is now in the early stages of human testing with the goal of producing a single-shot vaccine to immunize the Southeast Asian country’s population, said Chief Executive Officer Joseph Payne.
“The understanding is that because Singapore funded and helped us develop the vaccine, our intention is to definitely play a key role in their vaccination strategy,” Payne said in an interview Tuesday.
As it becomes clear that countries cannot fully re-open their economies safely before a vaccine is available, big and rich nations have secured early supply from major front-runner candidates by companies such as AstraZeneca Plc and Pfizer Inc. Other nations lower in the pecking order have taken matters into their own hands, with Thailand and India creating their own candidates, or in Singapore’s case, signing up with smaller pharmaceutical players.
While some international groups and a number of nations are promising to make vaccines affordable and accessible to all, doses will likely struggle to keep up with demand at first, putting those with early supply agreements at an advantage.
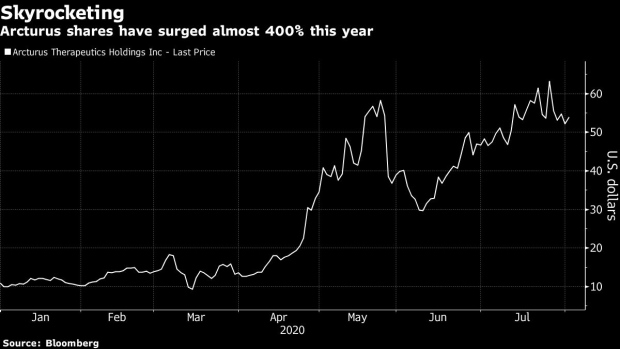
As with many vaccine makers seeking a shot to control the pandemic, Arcturus has seen its stock soar. The shares have surged nearly 400% this year.
It’s too soon to know if Arcturus’s vaccine will be effective, and Payne didn’t give a time frame for when it could reach the market. The vaccine, known as Lunar-Cov19, is one of approximately 20 candidates globally that have reached the clinical trial phase, he said. Singapore’s Health Sciences Authority granted approval in July for the vaccine to be tested in a study involving 108 healthy volunteers of varying ages.
The trial is due to be completed in October, with results to be available shortly after, according to Ooi Eng Eong, deputy director of Duke-NUS’s emerging infectious diseases program. The school is a partnership between Duke University in the U.S. and the National University of Singapore.
If the tests prove successful, a separate trial will be conducted among a larger group of individuals across countries with higher prevalence of the virus to determine efficacy, Payne said. He added that the company has also signed a supply agreement to deliver the vaccine to Israel.
More than 160 vaccines are currently under development globally. Moderna Inc. and China’s CanSino Biologics Inc. are among the leading candidates with shots in the advanced stages of human testing.
Single-Dose Approach
Arcturus and Duke-NUS aim to produce a single-dose vaccine. That’s in contrast with some of the front-runner candidates, which may require double doses as a vaccine’s efficacy wanes over time. Double shots are likely to prove costly and more complicated to distribute widely.
“A single dose will work so much better in terms of the logistics to get people protected against Covid-19,” Ooi said.
The Lunar-Cov19 vaccine is self-replicating and would extend the duration that an individual’s immune system is exposed to the antigen, hopefully triggering a sufficiently robust immune response over time, Ooi said.
Vaccine Production
Depending on the dose size, Arcturus and Duke-NUS aim to produce up to 30 million doses in the initial batch of vaccines. The company recently signed a production agreement with pharmaceutical company Catalent Inc. of Somerset, New Jersey.
The number of doses produced in the first batches would likely exceed the quantity required by Singapore and may be available for distribution to other countries, Ooi said.
©2020 Bloomberg L.P.