Dec 28, 2021
U.K. poised to clear Astra shot as need for vaccines grows
, Bloomberg News
Moderna's vaccine is a great additional tool and more stable: Infectious diseases consultant
The U.K. is poised to approve the COVID-19 vaccine produced by AstraZeneca Plc and the University of Oxford, giving the country another powerful tool to fight the pandemic as concern mounts over rising infections.
Britain’s drug regulator could clear the shot for use as early as this week, according to a person familiar with the matter, who asked not to be identified because the deliberations are confidential. AstraZeneca Chief Executive Officer Pascal Soriot and U.K. health officials had previously said they hoped for approval by the end of the year.
The go-ahead would come about three weeks after the U.K. became the first western country to begin vaccinations, using a shot from Pfizer Inc. and BioNTech SE that’s been administered to more than 600,000 Britons. Still, virus cases have surged in Britain amid concern about a new strain of the coronavirus that officials have said is more contagious.
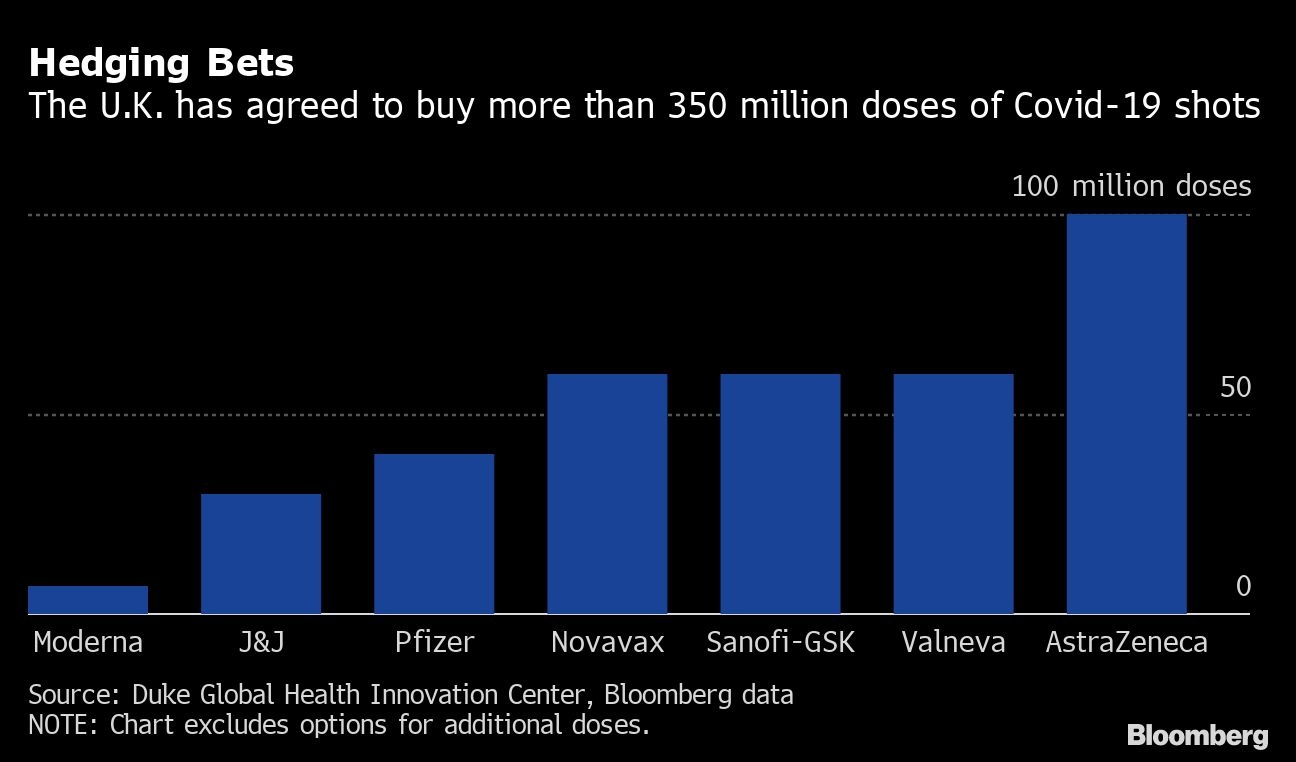
That prompted the government to reverse plans to ease restrictions over Christmas and tighten lockdowns across large parts of the country. Those measures could be eased at the end of February as the imminent approval of the AstraZeneca-Oxford shot permits the vaccination of as many as 15 million of the country’s most vulnerable people, the Mail on Sunday reported. The country’s health service would no longer be at risk of being overwhelmed by virus cases once that threshold is met, the newspaper said.
The Medicines and Healthcare Products Regulatory Agency will need time to carry out a review of the vaccine data, the Department of Health and Social Care said by email. Representatives for the MHRA and AstraZeneca declined to comment.
AstraZeneca’s American depository receipts rose as much as 3.6 per cent on Monday.
The AstraZeneca vaccine could facilitate a rapid ramp-up of vaccinations because it’s easier to transport and store than the Pfizer-BioNTech shot, requiring only refrigerator temperatures rather than deep freezing. It’s also less expensive to produce, so many developing countries -- along with the U.S. and the European Union -- have also signed deals for doses. The vaccine could be rolled out across Britain from Jan. 4, the Sunday Telegraph reported.
Trial Questions
U.K. approval would provide vindication for the AstraZeneca-Oxford shot, which has been slowed by questions about discrepancies in the trial results. Overall, these studies showed that the vaccine was less effective than shots from Pfizer and another developer, Moderna Inc. But a subset of the trial that showed better results resulted from a dosing mistake.
The U.K. has more riding on the domestically developed shot than the U.S. and some other countries because it won’t be able to get any of the Moderna vaccine until well into next year.
Soriot told the Sunday Times that new data will show the AstraZeneca vaccine is comparable to the 95 per cent effectiveness rate reported by the rival developers.
“We think we have figured out the winning formula and how to get efficacy that, after two doses, is up there with everybody else,” he told the newspaper. “I can’t tell you more because we will publish at some point.”
Soriot had previously said the vaccine was on track for large-scale vaccinations by the end of the year. England’s chief medical officer, Chris Whitty, had said he expected the MHRA to act by early next year or perhaps sooner.
Vaccination Sites
The final cut of data was submitted to regulators last Monday and the National Health Service has enlisted more than 10,000 medics and volunteers to administer the shot, the Telegraph reported. Mass vaccination centers at sports stadiums and conference venues are expected to begin in the second week of January, according to the paper.
Government officials will hold a meeting on the pandemic Monday after scientists warned that school closures may be necessary to slow the spread of the new Covid-19 variant, the newspaper said.
The country has been one of the hardest hit in Europe with more than 70,000 deaths, the most in the region after Italy. Much of the U.K. has been moved into the harshest Tier 4 restrictions, which prohibit household mixing and forced the closing of pubs, restaurants and many businesses, after the discovery earlier this month of the more contagious strain.
The rate at which the virus is increasing, known as the R number, is estimated at 1.1 to 1.3 as of Dec. 24, according to the latest government data. A reading above 1.0 indicates the spread of the virus is accelerating. The U.K. reported 34,693 more cases and 210 deaths on Dec. 26.
--With assistance from Suzi Ring.
