Aug 19, 2022
Europe Recommends Cutting Monkeypox Vaccine Dosage as Cases Rise
, Bloomberg News
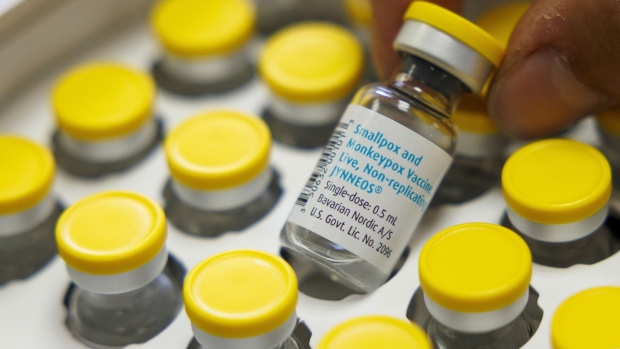
(Bloomberg) -- Europe’s drug regulator has recommended a different injection method and dosage for the monkeypox vaccine to increase the number of available shots as supplies run low and cases rise worldwide.
The European Medicines Agency Emergency Task Force said the Imvanex vaccine from Bavarian Nordic A/S can now be administered intradermally at a lower dose so that more people can be vaccinated.
An intradermal injection occurs between layers of skin, rather than under the skin, and will mean a single vial of the vaccine, also known as Jynneos, can supply five shots instead of just one.
The method is safe and used in other types of procedures such as tuberculosis tests, but it’s less common than subcutaneous vaccination and can be more difficult for health professionals to administer. The EMA warned an intradermal shot could trigger more local reactions such as longer-lasting redness and thickening of the skin.
Earlier this month, the US Food and Drug Administration approved emergency use of intradermal injections for the monkeypox vaccine. Both US and EU drug regulators cite a 2015 study showing little difference in efficacy of the shot between the two injection methods, though some health experts argue such evidence is insufficient.
Read More: Cutting Monkeypox Vaccine Dosage Is No Easy Fix, Officials Say
Monkeypox, now declared as a public health emergency of international concern by the World Health Organization, has infected more than 35,000 people across nearly 100 countries. The outbreak so far has disproportionately affected men who have sex with men and has spread primarily through close contact.
Bavarian Nordic Chief Executive Officer Paul Chaplin has expressed “reservations” about dose-sparing practices. He said such injection methods tend to cause more side effects and there’s limited safety data, in a letter addressed to US health authorities.
Countries that are hotspots of the monkeypox outbreaks are grappling to get vaccine supplies. The UK said earlier this week it is experiencing a temporary shortage and can’t book any more appointments until it receives extra doses later in September.
Meanwhile, Bavarian Nordic is seeking to outsource some production of the shot to help it meet surging demand from governments around the world. The company is said to have reached a deal with Michigan-based Grand River Aseptic Manufacturing Inc. to fill the Jynneos vaccine, Bloomberg reported earlier.
©2022 Bloomberg L.P.


