Jun 24, 2022
Moderna’s Covid Shot for Children and Teens Wins CDC Support
, Bloomberg News
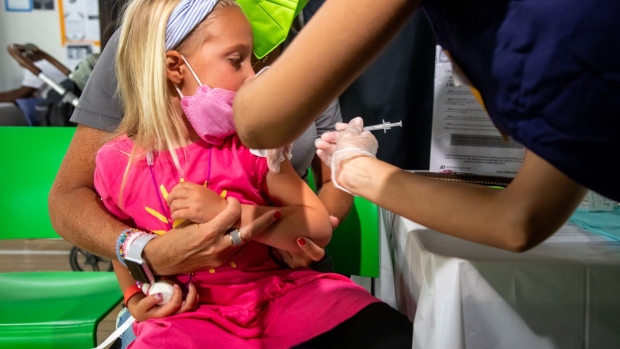
(Bloomberg) -- Children and teens across the US are now eligible to receive Moderna Inc.’s Covid-19 shots after the head of the Centers for Disease Control and Prevention granted the final clearance needed for injections to begin.
The recommendation by CDC Director Rochelle Walensky allows the use of two doses Moderna’s 50-microgram shot for children from 6 to 11 years old and two doses of its 100-microgram shot for children 12 to 17 years of age.
“Today, we have expanded the options available to families by recommending a second safe and effective vaccine for children ages 6 through 17 years,” Walensky said in a statement.
Until last week, when US regulators cleared the use of Moderna’s Covid shot for children and adolescents, only Pfizer’s vaccine was available for the age group. Authorization of Moderna’s shot for children 6 to 17 years old had been delayed by concerns about heart complications that have been seen in a relatively small number of recipients, mostly young men. Recent data suggest that the shot is effective in children and adolescents, with mostly mild to moderate side effects, according to the US Food and Drug Administration.
Among children ages five through 17 years of age, there have been more than 10 million Covid-19 cases, over 45,000 hospitalizations and over 600 deaths since the beginning of the pandemic, according to data presented Thursday at a meeting of CDC advisers. The panel of experts voted unanimously to recommend the Moderna vaccines.
“Covid can cause severe disease and death among children and adolescents, including those without underlying medical conditions,” CDC Epidemic Intelligence Service Officer Sara Oliver said at the meeting. “Future surges will continue to impact children, with unvaccinated children remaining at a higher risk of these severe outcomes.”
Still, the vaccination rate for children has been slow. Just 29% of American kids ages 5 to 11 are fully vaccinated, suggesting that some parents are hesitant or less motivated to inoculate their children.
The agency is trying to tackle the issue by making sure that information on the safety and effectiveness of these vaccines is available for parents. The agency has said it will host clinician education calls and parent webinars as well as publish educational materials on social media.
©2022 Bloomberg L.P.