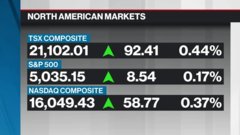
Dec 4, 2023
The Pentagon Wants to Root Out Shoddy Drugs. The FDA Is In Its Way.
, Bloomberg News

(Bloomberg) -- One morning in October, US Army Colonel Victor Suarez finished his usual morning workout — a 32-mile bike ride — and then sat down in his home office in Frederick, Maryland. When he opened his email, his stomach dropped.
Suarez spent his career getting medicines to military hospitals and combat troops, including those in Iraq and Afghanistan. He had recently sought out an independent lab to assess the quality of those drugs, in large part because he doubted the US Food and Drug Administration’s ability to police a supply chain now dominated by low-cost manufacturers in India and China. His inbox offered a glimpse of the first batch of test results.
They revealed that some generic versions of one important drug, given to soldiers who’ve lost limbs in combat, might not work. They could even cause kidney failure and seizures. The idea that already-wounded personnel might be facing an even more difficult recovery — all to save a dollar or two a pill — gutted Suarez. “Oh my God, what are we doing here?” he recalled thinking.
Still more infuriating was that doctors had long warned the FDA that some versions of the drug performed poorly. Known by the chemical name tacrolimus, it’s typically prescribed to organ-transplant patients. The lab Suarez engaged had started testing samples in early September. One of the generics assessed as problematic is manufactured in India by Intas Pharmaceuticals Ltd. It was only a couple of weeks later that the FDA announced, after more than a decade of study, that the Intas version wasn’t equivalent to the brand-name drug it sought to mimic. The outside lab had taken a month to flag similar problems.
As Suarez well knew, this isn’t just a problem for the military. Tacrolimus is just one example of the painful compromises at the heart of an American health-care system in love with — and increasingly suffering from — cheap generic drugs.
They’ve saved taxpayers and consumers hundreds of billions of dollars a year. But recalls related to drug manufacturing quality doubled in the US from 2018 to 2022. With almost 80% of FDA-registered generic production facilities located overseas, years can pass between inspections. Profit margins on generics are so thin that there are often only one or two suppliers of vital medications. As a result, quality issues are fueling shocking shortfalls in supply, which a US Senate committee will examine on Tuesday. Shortages in the US reached near-record highs this year and left cancer patients, among others, waiting for life-saving treatments. Generics are supposed to be affordable and safe. Instead, they’re cheap — but sometimes dangerous.
In February, US health authorities linked eye drops made in India to a rare bacterial strain that led to four deaths, blinded others and caused dozens of infections. Sold over the counter at major US drugstores, the drops came from a factory that had never been inspected by the FDA. Indian-made cough syrups laced with toxic industrial solvents have turned up over the past year and a half in 10 countries. They’ve been linked to the deaths of at least 140 children. The discovery of a probable carcinogen in blood pressure pills made in India and China five years ago is still driving recalls, with the same chemical appearing more recently in diabetes treatments.
The US reliance on India’s manufacturers is only growing as it turns away from China, a geopolitical rival. But in the effort to woo a strategic partner and ensure needed supplies, the FDA has been slow to address mounting evidence of flawed drugs. The regulator shut down a program of unannounced inspections in India almost a decade ago. When FDA inspectors do make it inside factories there, they’ve documented disturbing conditions, from barefoot workers in areas supposed to be sterile to widespread corner-cutting on tests.
Amid the scrutiny, Intas is becoming a familiar name. Maker of the tacrolimus that jumped out in the tests Suarez saw, it’s also an essential supplier of generics used to treat cancer. The company suspended production of them after FDA inspectors found that workers at one plant in India stashed shredded test results in garbage bags to hide evidence of shoddy manufacturing practices. (Intas said in a statement that it is “in the process of remediating the findings” and that it is “committed to providing safe and effective medicines.”) The subsequent shortages impacted care for one in 10 patients this year, the American Cancer Society says.
Concern about the nation's drug supply has reached the point that big hospitals, the Defense Department and Congress are raising questions about the FDA's ability to monitor it. The Pentagon in August chose an independent lab with which the FDA has publicly feuded, Valisure LLC, to test some of the generics available to millions of military personnel and their families, two years after Kaiser Permanente, a health system serving 12.7 million in the US, started a similar program with the lab. The efforts have run headlong into a major roadblock: the FDA itself. One might assume the US drug regulator, which dates to the 19th century Division of Chemistry, leads the mission of testing drugs. In fact, the FDA resists the idea of grading drugs by quality and rarely conducts tests of its own. Agency officials sought to block the Pentagon's nascent study, cast doubt on Valisure's methods and, according to multiple government officials, soured a Biden administration effort this year to introduce third-party testing more widely.
FDA spokesperson Jeremy Kahn said the agency “is continuously working to ensure that all drugs meet the highest quality standards with the health and well-being of Americans top of mind.” Agency officials have said many of the problems are a hangover from a decline in inspections during the pandemic and that a system based on spot checks and occasional reprimands is working.
The drawbacks of that approach were apparent in May, when an inspection of a second Intas facility in Ahmedabad, India, turned up more quality issues, including workers who made up favorable test results and ignored evidence of contamination. Inspectors had identified similar problems in 2019. Company officials suggested pausing production for the US to address the issues, according to a person familiar with the matter who declined to be identified discussing sensitive matters. The FDA, this person said, feared the fallout of even more shortages and advised against it.In November, after Bloomberg News asked an agency spokesperson about the decision, the FDA banned some exports from the plant to the US. But the ban didn’t apply to more than two dozen generics, including tacrolimus and 16 cancer drugs.
Robert Califf, the FDA commissioner, donned a garland of pink flowers on the steps of the US Embassy in New Delhi in September, smiling broadly for a photo with the American ambassador as he embarked on a trip to meet with government officials, drug company executives and academics. His subsequent blog post walked a line between pep talk and tough talk for India’s embattled generic drug industry. While noting the “global attention focused on serious instances of quality failures,” he said he’d also visited firms that are doing “a tremendous and reliable job” in a country that remains a critical partner.
Califf, who also led the FDA during the last year of the Obama administration, has returned to an agency in crisis. He and other officials were called before Congress last year to explain the FDA’s slow response to evidence of contamination in baby formula made in Michigan that was linked to infant deaths. Before that came its approval, in 2021, of a potentially lucrative drug for Alzheimer’s disease over the objections of a scientific advisory panel that questioned the drug’s effectiveness. The agency didn’t make him available to comment for this story.
The revolving door at the FDA, as at many other branches of government, is well-worn. At least four former chiefs of its office of compliance later became consultants to generic drugmakers. The head of its Center for Drug Evaluation and Research, Patrizia Cavazzoni, previously held senior positions at the drug giants Pfizer Inc. and Eli Lilly & Co. Like nine of 10 of his predecessors, Califf, a cardiologist, went on to work for drug companies or serve on their boards. Between his stints as FDA chief, Califf worked for Google parent Alphabet Inc.’s Verily Life Sciences unit and had advisory or board roles for at least seven other companies, including one developing Alzheimer’s treatments. (He has quit all of them since returning to the regulator.)
But the person who most shaped the modern FDA is Cavazzoni’s mentor, Janet Woodcock, 75, who said last month that she’s retiring after a 37-year career that included running the drug evaluation center and serving as acting commissioner, for a year until Califf’s appointment. Now principal deputy, she’s known to people in the industry as “shadow commissioner.” As the regulator has lurched from controversy to controversy in recent years, it is Woodcock’s mindset that has been preeminent. It boils down to encouraging manufacturers to fix problems themselves. “You cannot test products into compliance,” she said in an email. “The best quality is assured when manufacturers are dedicated to high quality.” Some former agency officials say this approach leaves the FDA reacting to problems instead of anticipating them. It also allows officials to shift blame, said Frank Yiannas, the former deputy commissioner for food policy and response, who described the baby formula crisis as a preventable tragedy exacerbated by the agency’s own mistakes. “There’s a fear at the FDA where nobody wants to be responsible for the supply chain,” he said. “The American public expects the government to do all that they can."
Woodcock laid out the agency’s “risk-based approach” two decades ago in a regulatory framework called “Pharmaceutical Quality for the 21st Century.” Ideally, the FDA would do as little as possible. She envisioned “a maximally efficient, agile, flexible manufacturing sector that reliably produces high-quality drug products without extensive regulatory oversight.”The offshoring of US pharmaceutical manufacturing to India and China was then just getting started, but some had already warned about losing control. A 1998 report by what’s now called the Government Accountability Office cited concern about the FDA’s “ability to ensure the safety and quality of the increasing volume of foreign-produced drugs imported daily into the United States.”Those worries materialized a decade later, when a contaminated blood thinner made in China led to hundreds of deaths in the US. That same year, the FDA opened its first offices in Beijing and New Delhi. The agency opened a second India office in Mumbai the following year, and had plans to hire 19 staffers in the country.
In a case that again jolted the generics market, the US unit of India’s Ranbaxy Laboratories Ltd. in 2013 pleaded guilty to felony charges of selling adulterated drugs and lying about it to the FDA. Ranbaxy paid $500 million to settle the case, which further exposed the flaws of an FDA inspection regime organized to monitor a domestic manufacturing base that was rapidly disappearing. While surprise inspections were easy to do in the US, the overseas inspectors had to announce their arrival weeks or months in advance.
The next year, following the Ranbaxy scandal, the FDA started a pilot program for unannounced inspections in India. It uncovered more troubling signs that factories there weren't just dirty and under-equipped; workers were routinely hiding sensitive documents.
But the program lasted only about a year, until a July 2015 email from Alonza Cruse, then acting director of the FDA’s Office of Pharmaceutical Quality Operations. “The pilot will end immediately,” Cruse wrote in the email, obtained by Bloomberg. No explanation. The agency later told the GAO, the investigative arm of Congress, that its staff hadn’t developed any metrics on which to evaluate the pilot’s success.
Two former employees, who didn’t want to be identified discussing internal matters, said FDA leaders were also concerned that unannounced inspections could undermine efforts to deepen relations with India, a year after the nationalist government of Prime Minister Narendra Modi came to power. Asked about those claims, FDA spokesperson Kahn said the agency’s “collaboration with India highlights continued advancement of the production and availability of medical products that both countries and the entire world rely upon.”
By 2019, India’s manufacturing clout had grown to the point that the country had more FDA-registered generic drug facilities than the US, according to a Bloomberg analysis of agency data. (India had 30% as of Oct. 1, compared with 22% for the US and 13% for China.) Yet the FDA’s footprint in the country has shrunk. After closing the Mumbai office in 2016, it had a drug inspection staff that recently numbered four in New Delhi. The office primarily focuses on building relationships in government and industry rather than compliance.
Just before the pandemic, Valisure, a tiny lab in New Haven, Connecticut, started gaining customers – and embarrassing the FDA – by doing something people expect the regulator already does: test drugs.
The lab’s co-founder, Adam Clark-Joseph, had suffered complications from an anticonvulsant drug he was taking. He’d connected with a friend from Yale University, a molecular biologist named David Light, and they’d become convinced there was a business in screening medications. (Bloomberg commissioned Valisure this year to test Indian-made cough syrups obtained from six countries; one test found unsafe levels of toxic chemicals in a cold medication sold in Iraq and prompted a recall.)
The FDA doesn’t regularly test either ingredients or the finished products. During inspections, it primarily checks to see how well companies follow manufacturing procedures rather than conduct its own sampling. When the regulator does find problems, it typically relies on the company to voluntarily take action to address the root cause.
At first, Valisure operated as a pharmacy and tested the medications it dispensed. Light said about 10% had problems, such as contamination or the lack of an active ingredient. Then came a bombshell finding in 2019 that Zantac, the widely used heartburn drug, was contaminated with a chemical that likely causes cancer. A massive recall followed. Valisure also found leukemia-causing benzene in hand sanitizers, sunscreens and antiperspirants. Again, companies withdrew the products — and the FDA had to answer questions from Congress about how an obscure lab had sounded the alarm first.
Months after the Zantac revelation, the pandemic forced a near-halt in the FDA’s inspections. Agency officials still found time, in May 2021, to send a team into Valisure’s offices. In public statements about the lab, the regulator had grown increasingly critical, telling reporters its own testing revealed discrepancies from Valisure’s. Though the agency also found unsafe levels of the probable carcinogen NDMA in Zantac, it said the lab's tests had inflated them.The inspection had been ordered by “HQ,” agency headquarters in Silver Spring, Maryland, a high-ranking FDA investigator wrote in an email obtained through a public records request.
Staffers on the ground appeared mystified by the assignment. Over the following weeks, according to the emails, the FDA team repeatedly conveyed to top officials that nothing at the lab indicated the testing it did was subject to agency oversight. Valisure doesn’t make drugs, and its contracts with clients say none of its testing should be used for any regulatory purpose. Twice, the team tried to close the inspection. They suggested that a discussion about some technical violations they’d spotted, not an official admonition, would suffice. But Francis Godwin, head of the Office of Manufacturing Quality, insisted on a tougher report, the emails show. (Godwin didn’t respond to a request for comment.)
Nineteen months after the initial inspection, the FDA published an eight-page letter enumerating flaws in data collection and equipment that applied if Valisure were to do regulatory work — which, the letter acknowledged, it didn’t. The letter said Valisure hadn’t documented the “accuracy” and “repeatability” of its results, among other concerns. The FDA’s Kahn said the agency isn’t opposed to additional screening of drugs but added that such measures must be “backed by validated testing methods, scientific research and expertise.”
In the view of some current and former staffers, who requested anonymity to protect their careers, the episode amounted to a hit job meant to clip the momentum of a company whose tests had called into question the regulator’s effectiveness. Others who have raised concerns about drugs say they’ve often been met with silence. “The FDA has a story and the story is: ‘We’re the FDA, we know what we’re doing. Trust us,’” said Joe Graedon, co-founder of the People’s Pharmacy, a consumer health organization that publishes complaints about generics. “Anything that interferes with that story or criticizes that story is generally not welcome.”
All of this helps to explain why independent testing, and Victor Suarez’s collaboration with Valisure at the Department of Defense in particular, touched off such an intense bureaucratic struggle this year.
As tension with China has increased, India, which accounts for one-fifth of the world’s generic drug exports, is seen by US officials as a more secure source of supply. “We’ve done a big push with India,” said Neera Tanden, President Joe Biden’s domestic policy adviser. After Modi met with Biden at the White House in June, they issued a joint statement welcoming “deeper collaboration” in pharmaceuticals and calling their two countries “among the closest partners in the world.”
The relationship has survived some recent stress. A year ago, FDA inspectors issued a 36-page report describing the efforts of employees at the first Intas factory they visited to hide test results from them. One employee “rushed and tore apart” printouts and threw them into a trash bag, then poured acid onto them.
The company makes about 50% of the US supply of cisplatin, a widely used chemotherapy drug. After Intas ceased production at that factory to address issues raised by the inspectors, it led to a shortage of both cisplatin and another chemotherapy drug called carboplatin. That left cancer patients across the country scrambling to find hospitals able to secure the drugs, or even delaying treatment.
The shortages have embarrassed an administration that promised an ambitious “cancer moonshot” to cut death rates in half and now finds itself simply trying to ensure access to existing drugs. Early this year, Biden officials led by Susan Rice, then his domestic policy adviser, began crafting a $25 billion package to restore US drug manufacturing and improve visibility into supplies, modeled on the CHIPS Act for semiconductors, according to people familiar with the matter who asked for anonymity to share plans that were not public. Among the steps to improve quality, they consulted with the FDA about implementing third-party testing of the type Valisure does.
After Bloomberg later reported the Pentagon's plans to test drugs with Valisure, FDA officials told the White House they felt betrayed, according to these people. The agency’s leadership suggested that the testing, though still only a pilot, was a direct assault on its reputation and performance, sowing distrust in the products it clears, the people said. It was an awkward moment because Rice, the official who’d been most focused on policing supplies from India and China, had just left her post. Momentum for sweeping action stalled. Rice didn't respond to requests for comment.Tanden, who replaced Rice in the role, said the administration continues working with the FDA to ensure the availability and quality of medicines and is open to various ideas, including different measures of quality. She added that the White House tried to interest lawmakers in investing billions to shore up the pharmaceutical supply chain, but Congressional interest waned. The FDA’s Kahn said it's open to “meaningful solutions” in “collaboration with our cross-government partners.”
In August, Suarez, 50, went on stage with Valisure’s Light at a conference of medical supply experts in Orlando. Congress had told the Pentagon a year ago to identify threats to its pharmaceutical supplies. Suarez got involved in the project as something of a swan song to a career of supporting vaccine development and managing medical acquisition and logistics for 25 military facilities. He retired last week and is now a consultant who advises health-care clients on product and supply chain issues. As Suarez announced the pact with Light that day in Orlando, he said it would help end what’s in effect an “honor system” that leaves consumers in the dark.
That same month, Suarez said, he heard of blowback through his command chain. Califf met with one of his superiors, Assistant Secretary of Defense for Health Affairs Lester Martinez-Lopez, and raised concerns about the Pentagon study and Valisure’s testing methods. Martinez-Lopez informed Califf the Pentagon would move forward, according to Suarez. Nicole Schwegman, a Defense Department spokesperson, said it “appreciates the FDA’s insight” and noted that Martinez-Lopez is required to report on risks to the pharmaceutical supply chain. The FDA’s Kahn didn’t respond to questions about the meeting.
To sustain support, Suarez also briefed Pentagon leaders about Valisure's preliminary results for a few medications, including the flawed tacrolimus. A chart that compared prices alongside quality scores showed how cheap isn’t always best. “It was just a jaw-dropping moment,” he said. The Valisure pilot is testing a dozen drugs, each with multiple manufacturers, among them blood pressure medicines and antidepressants. The lab uses commercial samples obtained from distributors, not manufacturers. The tests measure dosages, as well as potential contaminants. Results are graded by an outside panel of experts, who assign quality scores.
Doctors had expressed doubts about some generic versions of tacrolimus soon after they were introduced in 2009. The FDA responded two years later by designating it a “narrow therapeutic index drug,” signaling that small differences in dosages can have a big impact on patients. Over the next several years, the agency has said, it funded “a number of studies” to continue investigating. Finally, in September, the agency said the Intas version isn’t equivalent — but let it stay on the market.
Last month, the Pentagon gave the Valisure project another vote of confidence by transferring it to the Uniformed Services University of the Health Sciences, which conducts research for the military. The institution will add dozens of additional medicines to the study and analyze the results over the next two years. Suarez said he hopes big drug buyers like Medicare and the Veterans Administration adopt the same approach — which might finally change the economics of the generic drug market to reward quality. “Hopefully the FDA will become part of that,” Valisure’s Light said, “but it’s happening without them anyway.”
In the European Union, independent testing has long been standard, with a network of 70 labs that sample medications both before they’re released and after they’re in use. The FDA’s Woodcock said European authorities also rely on inspections and work closely with the FDA. Tests might motivate “certain manufacturers to make sure their products at least pass,” she wrote in an email. “But people who know a lot about this will tell you that testing per se is not a magic bullet.”
After abandoning pursuit of the $25 billion legislative package, the Biden administration last week tapped the Defense Production Act to enable some domestic investment in essential medicines, starting with $35 million for sterile injectable drugs. It also added a new post outside the FDA for a “supply chain resilience and shortage coordinator.” Biden adviser Tanden said the administration is exploring other ways of using the clout of the US government “as a giant purchaser of drugs” to encourage manufacturers to prioritize quality and availability. Prompted by Congress, the FDA resumed unannounced inspections in India last year and China more recently.
What’s missing is a shift in the mindset of the regulator. Not long after FDA commissioner Califf returned from India, he spoke at an October conference for the generic drug industry in North Bethesda, Maryland. Asked about the idea of health systems doing independent tests, he responded: “I might say there are better ways to spend your money.”
--With assistance from Laura Bejder Jensen, Ike Swetlitz and Swati Gupta.
©2023 Bloomberg L.P.