Mar 6, 2024
WuXi AppTec Drops as US Senate Panel Advances Bill Banning Deals
, Bloomberg News
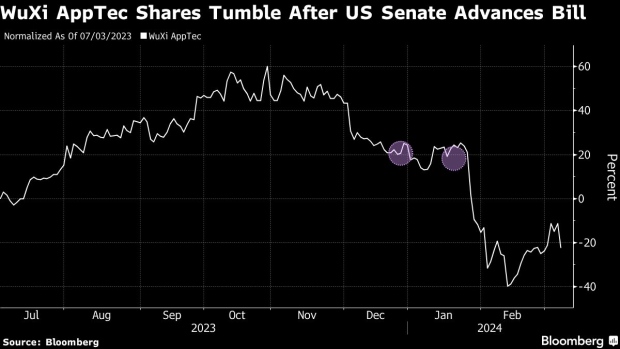
(Bloomberg) -- WuXi AppTec Co. tumbled after a US Senate committee advanced a bill that may ban Chinese biotech firms from accessing federal contracts and largely cut off the biopharmaceutical company from the market that generates more than half of its revenue.
Shares of WuXi AppTec plunged as much as 24%, while its sister company WuXi Biologics Cayman Inc., which is also named in the bill, dropped 23%. Both stocks have lost more than 40% so far this year, making them the worst performers on the benchmark Hang Seng Index.
The legislation adds to the geopolitical risk faced by Chinese stocks, already weighed down by the country’s sluggish economy. The bill’s passage would add to China’s mounting frustration over the US imposition of trade curbs in their battle for supremacy in advanced technology, such as semiconductors, artificial intelligence and biotechnology.
The frustration boiled over Thursday as China’s Foreign Minister Wang Yi blasted the US for its tactics. “If the US is obsessed with suppressing China, it will eventually harm itself,” Wang said.
Geopolitical tensions also continue to weigh on China’s stock markets, which recently have shown some signs of life after a year of deep downturn. The two companies dragged down the Hang Seng Composite Index’s healthcare gauge as much as 5.3%.
The US Senate Committee on Homeland Security and Governmental Affairs’ mark-up Wednesday sends the bill to the Senate for a floor vote. The bill — which would bar some biotechnology firms “of concern” with alleged ties to a foreign adversary from federal contracts — still must pass both chambers of Congress and get the president’s signature before becoming law.
US Market
“We believe the decision of the Senate committee may adversely impact China’s CROs,” Jialin Zhang, head of China healthcare research at Nomura International HK Ltd, wrote in a note, referring to contract research organizations. “The US is a vital market for Chinese CRO.”
If the rare bipartisan legislation goes through as currently crafted, drugs already in short supply, from the blockbuster GLP-1 agonists to advanced cancer treatments, will become even harder to produce.
WuXi AppTec, which gets about 66% of its revenue from the US, said in disclosures that it works with the world’s 20 largest pharmaceutical companies. And many of its customers, including Eli Lilly & Co., produce drugs purchased in bulk by the government health care insurance programs in the US, such as Medicare and Medicaid.
Read more: Weight Loss Drugs Threatened by US Effort to Contain China
After the Senate markup, WuXi AppTec said in a statement Thursday that it doesn’t have a human genomics business, and promised to continue engagement with relevant stakeholders involved in the bill. WuXi Biologics also reiterated in a statement Thursday that it doesn’t pose any national security risk to the US or any other countries.
Senator Gary Peters from Michigan, the bill’s sponsor and chairman of the Senate Homeland Security and Governmental Affairs Committee, is considering revisions that would allow existing contracts to remain in place, according to a person familiar with the discussions. Other options include removing the names of the Chinese biotechnology companies from the legislation.
Read: JPMorgan EM Equity Exits Wuxi Biologics, Buys More MediaTek
US drugmakers could try to separate their supply chains from Chinese biotech companies just on products involving government or US contracts, but that would be arduous and uneconomical. WuXi AppTec and WuXi Biologics both have manufacturing facilities that have been approved by US regulators.
“Geopolitical uncertainties triggered by the US biosecure bills may weigh on near-term valuations in this US election year,” Morgan Stanley analysts Sean Wu and Daisy Cheng wrote in a note dated March 4.
--With assistance from Michelle Fay Cortez and Madison Muller.
(Updates with company statements 10th paragraph)
©2024 Bloomberg L.P.