Dec 6, 2023
Can Wegovy Fight Alcoholism? For Big Pharma, This Isn’t a Priority
, Bloomberg News
(Bloomberg) -- Every week, about two dozen patients come to a small room in Frederiksberg Hospital, a maze of old red-brick buildings in central Copenhagen. They are blindfolded and told to insert earphones with music. Then a nurse injects them with what they hope is the blockbuster weight-loss drug Wegovy.
The patients are trying to shed an addiction, not unwanted pounds. They are volunteers in one of a growing number of studies begun in the US and Denmark this year to see whether Wegovy can treat alcoholism.
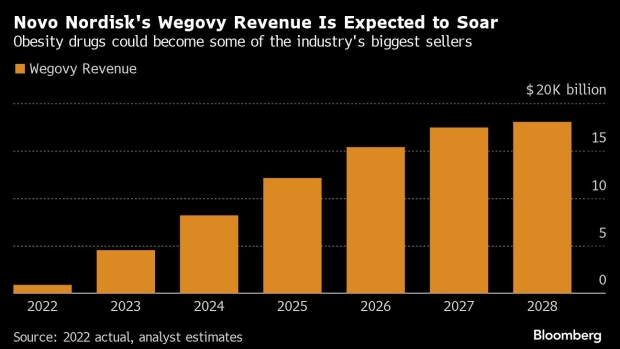
Researchers are pushing the boundaries on a class of drugs that became famous for helping people lose weight, testing the shots across a spectrum of disorders from Alzheimer's disease to sleep apnea. The market for obesity and diabetes will probably soar above $140 billion by 2032, analysts at J.P. Morgan predict, with the potential for even more if the drugs prove medically versatile.
But in the case of alcoholism, Novo Nordisk A/S, the Danish drugmaker behind Wegovy, isn’t involved even though one study is in its hometown. It didn’t agree to supply scientists with the medicines. Neither is Eli Lilly & Co., which makes a similar product. Big Pharma’s reluctance underscores why no new treatment has been approved for alcoholism in almost two decades: the commercial potential is uncertain, and pitfalls abound.
These medicines typically “aren't big moneymakers," said Christian Hendershot, a clinical psychologist and professor at the UNC School of Medicine in North Carolina. “Some drug developers don’t want to wade into this territory. It’s a vexing problem for the field.’’
Only three new treatments have been approved in the past seven decades in the US for alcohol-use disorder – the medical term for alcoholism – according to Hendershot, compared with 40 to 50 for diabetes.
The group that shows up weekly at the Copenhagen hospital belies some common stereotypes. The volunteers tend to have professional jobs; some work in management positions and arrive for their session in expensive cars. None have dropped out so far, an unusual feature that speaks to their motivation, according to one of the nurses involved in the study.
Some patients report lower appetite and skipping meals – a sign they may be getting the real Wegovy, whose celebrity status has vaulted Novo into the limelight and turned it into Europe’s most valuable company. Novo’s shares have risen 45% this year, though they slipped about 1% on Wednesday.
The study results so far have given one 53-year-old study participant, a grandmother, hope of stopping a familial chain of alcoholism that led to her estrangement from her own mother. “I said to myself, never again,” she said. “It should end now.”
When asked about its lack of involvement, Novo says that alcohol-use disorder isn’t one of its focus areas. But there could be other unspoken considerations behind the choice to remain on the sidelines. Anders Fink-Jensen, the professor who oversees the study at the University of Copenhagen, describes the patients as a “fragile group” who are likely to have other illnesses, precipitating safety concerns that could taint the medicine’s image.
“I can understand that to a certain extent,” he said in an interview. “If something happened, it could close down everything.”Another deterrent is the number of people who will end up using the medicine for addiction, which will likely be low, especially considering its side effects and the barriers that US insurers tend to place on access, according to Lauren Finke, a policy director at the Kennedy Forum.
“These are not going to be the money-making drugs for companies and they know that,” she said.
The way these treatments work in the body is fueling researchers’ hopes about their versatility. The active ingredient in Wegovy and its sister drug Ozempic, mimics a gut hormone called GLP-1 that the body produces immediately after eating.
While natural GLP-1 disappears quickly after a meal, the once-a-week injected pharmaceutical version lingers at very high levels for days. The drugs slow the movement of food through the gut, but their impact is far broader. Among the many places in the body where GLP-1 receptors are found is the brain, where the medicines are thought to affect dopamine, a neurotransmitter that helps regulate the flood of pleasure that comes from sex, a good meal or a glass of wine.
“It interferes with the reward system,” said Jens Juul Holst, a professor at the University of Copenhagen who made key early discoveries about GLP-1. “It’s extremely exciting.” Holst is affiliated with the Novo Nordisk Foundation, the namesake company's controlling shareholder, which is helping to fund the Copenhagen team’s research.
Patients have been telling their doctors about a diminished desire to drink alcohol ever since the first GLP-1 medicines went on the market more than a decade ago. In studies with animals, the treatments have been shown to reduce alcohol, cocaine, amphetamine and nicotine use as well as decreasing food intake. Danish researchers even found that two older drugs from this class reduced alcohol consumption in a population of hard-drinking vervet monkeys on the Caribbean island of St. Kitts.
But the few studies that tested the older, weaker medicines on alcohol-dependent people have been less conclusive. In the most ambitious one so far, published last year and run by the Copenhagen team, researchers were surprised to find that although people drank less when they took an older drug called exenatide, they didn’t reduce their drinking any more than those who had a placebo and therapy. However, scans showed less activation in the brain’s reward system when people who got the drug looked at alcohol images, potentially a sign that it was having an effect, said Mette Kruse Klausen, who helped run the study as her doctoral dissertation.
There was another silver lining: among patients with obesity, the drug seemed to perform better. But the trial hadn’t recruited enough people in that category for doctors to be certain the results weren’t a fluke. Another recent case study showed promising results for just six people. These patients were given semaglutide — the active ingredient in both Wegovy and Ozempic — for weight loss and suffered from alcohol-use disorder. They all saw a significant reduction in their symptoms.In the new Danish research, volunteers must have a body mass index of at least 30, says Klausen, who’s now helping to lead the Wegovy trial. The people enrolling also have a different profile, with more resources, and for most, this is the first time they’ve sought treatment, she said. They will get either Wegovy or a placebo, along with therapy, for six months.That can be a long time for someone with an addiction. In alcoholism studies, people often make a strong start, and it’s common to see a transformation in the first three months, according to Klausen; it’s making that change stick that’s more challenging. So the research team created a rule that volunteers are only allowed to miss five visits. Klausen originally expected about 40% of the patients to drop out, although as of late November, five months into the test, no one had.
In the US, at least four alcoholism studies are underway or will soon begin. Most are structured similarly – the American and Danish teams said they’ve been talking frequently since they all met over dinner at a medical conference in Washington state this past June.
"It's been the most exciting time," said Lorenzo Leggio, the clinical director of the US National Institute on Drug Abuse. "There's a lot of momentum, a lot of interest in this target."
In addition to measuring alcohol consumption by asking patients questions and performing blood and urine tests, Leggio’s team plans to expose participants to different stimuli, such as a bar-like room or cafeteria setting, to look for changes in cravings.
The economic burden of alcohol-use disorder is estimated to be approximately $250 billion across the US, according to a 2010 study. Just 1.6% of the more than 14.1 million adults with alcoholism were prescribed medications to help treat it, according to a 2019 survey backed by NIH, reflecting the dearth of options.
Novo recently shut down an early-stage project using RNA technology that had been begun by Dicerna, a biotech it acquired in 2021. If Novo were to start its own work in alcohol-use disorder, it would probably use an experimental medicine called CagriSema, not Wegovy, development chief Martin Lange told investors in early November.
Rival Eli Lilly, which is introducing a Wegovy competitor under the name Zepbound, declined to comment on its decision-making on alcoholism, saying only that it’s evaluating where its drug may be valuable beyond current uses in diabetes and obesity. However, the company hasn’t been responsive to requests from outside researchers for proposed alcohol-use disorder trials, said Joseph Schacht, an addiction psychologist at the University of Colorado School of Medicine.
Without the drugmakers’ backing, “the current market cost of these medications is a major impediment to studying them,” according to Schacht. At his organization’s research pharmacy, a month’s worth of semaglutide – the active ingredient in Wegovy – costs about $1,300, he said, meaning that medicine for a six-month trial of 150 patients would cost almost $600,000. The annual budget for a National Institutes of Health research grant, which includes personnel, labs and participant compensation as well as drugs, is typically capped at $500,000, he said.
The drugmakers’ lack of collaboration also creates a practical hurdle for researchers: they don’t get the injection pens the companies use in their own trials to keep both patients and doctors from knowing who’s getting the real drug and who gets a placebo. That’s why Klausen at the University of Copenhagen decided to bring patients into the clinic once a week for blindfolded injections. Schacht opted to use the pill form of Novo’s semaglutide, called Rybelsus, because it’s easier to mask the placebo.
Most of the testimonials from social media about Wegovy’s effectiveness as a curb to alcohol consumption are from people who drink socially. There may be different forces at play for people who have alcohol-use disorder, according to Fink-Jensen, the University of Copenhagen professor.
“Part of being addicted is that you have this, you know, stereotyped way of thinking about getting more drugs and getting more alcohol, and it's not really coupled to the rewards,” Fink-Jensen said. Some patients get medication to decrease their cravings and “can register that they are less interested, but they basically drink the same,” he said. Habit can also play a role, if buying a bottle is part of a patient’s daily routine. “It’s not necessarily coupled directly to desire.”
Another unanswered question is whether these drugs can be used short-term, like other treatments for alcohol-use disorder, or whether they'll need to be taken for the rest of someone's life as is the case for diabetes and obesity. Researchers don't yet know if a person's urge to drink will come back as soon as they stop taking them.
Among Klausen’s patients, the grandmother who lives in Hellerup, the affluent Copenhagen suburb where the Novo Nordisk Foundation is based, is counting on her own willpower after the trial is over. The woman, who asked not to be identified because friends and co-workers don’t know she is an alcoholic, used to come home at night from her stressful job as a manager and drink – first a glass or two of wine to unwind, and ultimately one or two bottles. She decided to make a change after a long talk with her oldest daughter.
By mid-November she was halfway through the study, stopping by Klausen’s lab for her shots every Thursday at 7 a.m. on her way to work. She doesn’t know whether she’s getting Wegovy, but she feels the treatment is effective. She has lost 11 kilograms and stopped drinking completely.
“It feels so good,” she said. “I got my old life back.” Will Novo Nordisk shares continue to rally, bringing the company's valuation up to $1 trillion? Share your views in our MLIV Pulse survey about the 2024 outlook. Click here.
--With assistance from Sanne Wass and James Cone.
(Updates with shares in eighth paragraph. An earlier version corrected the units in chart.)
©2023 Bloomberg L.P.


