Jul 31, 2023
The Psychedelics Stock Universe Is Shrinking, Despite the Hype
, Bloomberg News
(Bloomberg) -- Psychedelics companies aren’t living up to the hype, and are starting to consolidate as a flood of money into companies seeking to win regulatory approval of drugs like LSD, psilocybin and ayahuasca dries up.
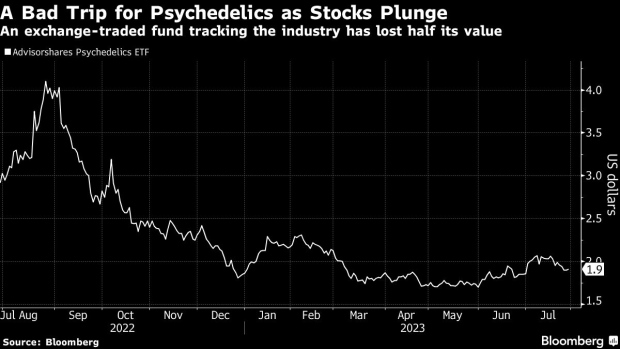
The psychedelics renaissance fueled by Michael Pollan’s 2018 book How to Change Your Mind is still going strong, with pop culture references and underground use thriving. Legal change has set the stage for some growth: In the past few years, Oregon has set up a framework for a legal industry, and Colorado has decriminalized such drugs. Yet most psychedelics companies are trying to get them approved through clinical trials. So far there are no major breakthroughs, and stocks have suffered: The Advisorshares Psychedelics ETF is down around 53% from its 365-day high last August.
The disconnect between lobbying visions and reality in psychedelics has once-high-flying companies now on the verge of failing to make payroll, said Cody Shandraw, a managing partner at Ambria Capital, which has invested in the space since 2019.
“When you look at any emerging industry, there’s a life cycle,” Shandraw said in a phone interview. “Now we’re in the shakeout stage.” He predicts that around half the companies that have gone public since 2019 are in the process of selling intellectual property or shutting down, and expects investments in the industry this year of around $400 million to $500 million — potentially even lower than in 2022.
According to an estimate based on data from Crunchbase and other sources, investments in the industry ramped up from just $62 million in 2019 to $617 million in 2020, and $1.6 billion in 2021 — and then faded to just $570 million last year.
Shandraw thinks many companies will go bankrupt, and while some will merge with other distressed companies or form joint ventures, only those with strong IP will survive.
Mirrored Trajectory
Psychedelics’ trajectory mirrors that of the broader biotech sector, which has seen a pivot away from risky assets. But psychedelic drugs — or novel variations on their active molecules — are also unique.
As cultural interest fueled by Netflix shows such as Have a Good Trip and Fantastic Fungi fed into the hype around psychedelics, many companies were able to go public even at the preclinical stage, leading to an overcrowded field. Not all of those companies have enough funding to get through clinical trials. The US Congressional Budget Office estimates it costs $474 million for preclinical work on a pharmaceutical drug, and $1.1 billion more for clinical trials, and that only 12% of drugs that reach the clinical phase actually go to market.
Other companies in psychedelics that are so-called infrastructure plays — developing retreats or locations where the drugs might one day be administered — have also floundered. Field Trip Health, which had ketamine clinics where it hoped one day other drugs like MDMA or psilocybin would be offered, recently sought creditor protection in Canada.
“The nonbiotech names, like ketamine infusion clinics, are struggling to sell the dream as investors have now seen several years of their operating performance,” said Chris Yetter, founder of Dumont Global, a fund that invests in cannabis and psychedelics — sometimes with a short position.
Late 2024
Shandraw said he sees hope in late 2024, when many companies will see midstage results from clinical trials. That’s also when many expect some news from the Multidisciplinary Association for Psychedelic Studies, known as MAPS, on MDMA, or ecstasy, and its attempt to get US Food and Drug Administration approval for its use to treat post-traumatic stress disorder.
The industry is also optimistic about the potential of traditional pharmaceutical companies to make purchases, often citing the example of Otsuka Pharmaceutical Co.’s investment in Mindset Pharma Inc. Otsuka, based in Tokyo, makes drugs including Abilify, to treat schizophrenia.
One company already seizing on deal-making opportunity is Lucy Scientific Discovery Inc. This month, Lucy acquired Wesana Health Holdings Inc.’s assets for a drug candidate that would combine psilocybin and cannabidiol, or CBD, to treat major depressive disorder, migraines and other conditions.
Lucy isn’t just interested in intellectual property. In June it also offered to buy Pasithea Therapeutics Corp. for a 142% premium to its then-35-cent stock price, to take advantage of the company’s $30 million of cash. Lucy Chief Executive Officer Richard Nanula said the plan was to buy out Pasithea’s current investors with half the cash, and keep the rest for Lucy.
Lucy’s plan was to then buy more psychedelics companies that trade for less than their cash value. It wanted to broaden from its current manufacturing focus to become a company like Atai Life Sciences NV, which has a portfolio of several drug candidates.
Last week, Pasithea rejected the bid. “The Lucy proposal does not reflect Pasithea’s strategic value and prospects for continued growth,” the company said in a statement.
©2023 Bloomberg L.P.





