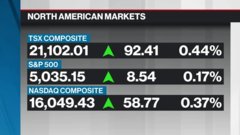
Jan 10, 2023
Pandemic Prodigy BioNTech Faces Crucial Year in Cancer
, Bloomberg News
(Bloomberg) -- BioNTech SE Chief Executive Officer Ugur Sahin’s office window looks onto a four-story stack of prefab labs and desk space built to house an overflow of workers.
Like its headquarters in Mainz, a short walk from the Rhine River, BioNTech is a company under construction. After catapulting to success with the best-selling messenger RNA vaccine for Covid-19 it developed with Pfizer Inc., the German biotech clinched its biggest takeover to date on Tuesday to embed artificial intelligence in its research, development and manufacturing.
Now Sahin and his team must prove they can make BioNTech into something more than a pandemic prodigy, delivering medicines that innovate in one of the pharmaceutical industry’s most competitive fields: cancer.
This year is “about the ability to execute,” Sahin said in an interview in late December. “That’s the most important aspect.”
The results of two crucial mid-stage trials will be released this year. BioNTech last week made a deal with the UK that gives it quicker access to patients to test these medicines. And the InstaDeep Ltd. takeover announcement comes as Sahin attends the JP Morgan Healthcare Conference, the industry’s top meeting to network with potential partners and seal deals.
BioNTech is vying with rival Moderna Inc. for supremacy in the mRNA field, seeking to use the technology to treat cancer as well as a range of infectious diseases including HIV and seasonal flu. Unlike Moderna, BioNTech is also investing in a plethora of other new technologies, from cell therapies to antibodies.
Cancer Test
“It’s actually part of our DNA,” said Ozlem Tureci, BioNTech’s chief medical officer and Sahin’s wife. “We are not a company which has a monolithic platform and sticks to it.”
A success in cancer would be most significant this year — in particular for an experimental melanoma mRNA vaccine BioNTech is testing together with Swiss drugmaker Roche Holding AG, according to Sam Fazeli, a Bloomberg Intelligence analyst.
Moderna set a high bar in melanoma, the most serious type of skin cancer, with excellent results in December.
It may be harder for BioNTech to similarly shine, because it’s treating patients whose tumors have spread and who haven’t yet had other treatment, whereas the US company gave its medicine to people who’d had surgery to excise their cancer.
“If first-line melanoma doesn’t work out, the stock could get badly hit,” Fazeli said. “The assumption will be that Moderna has something that these guys don’t, even though that’s not 100% true because the trials are different.”
Proof of Concept
If the gamble succeeds, however, Sahin and Tureci said success could be a stepping stone to many more trials.
Treating melanoma after it has spread “is a much higher hurdle,” Sahin said. But it could provide a rationale for using mRNA cancer treatment as an initial step for other types of tumors if it works. “This is sort of a proof of concept.”
The company will also make decisions this year about final-stage trials that could enable regulatory approval in 2025 and 2026 for some of its experimental cancer drugs, he said. Some of that work will be run in the UK under the partnership announced last Friday.
BioNTech’s most advanced product candidate is another mRNA vaccine, a flu shot it’s working on together with Pfizer. Late-stage trial results on that project are due this year. The market could be worth $10 billion by 2030, Pfizer said in a recent investor presentation. The partners are also working on a flu-Covid combination shot that’s not as far along in development.
Drying Up
As BioNTech faces the challenge of proving itself beyond Covid, the revenue stream that has spurred the company’s growth is drying up.
Revenue will likely drop to 9.2 billion euros ($9.86 billion) this year from an estimated 16.5 billion euros in 2022, according to analysts surveyed by Bloomberg. The company splits Covid vaccine revenue with Pfizer.
The company’s American depositary receipts have lost about a third of their value in the past 12 months as the omicron variant helped usher in an mindset of living with the pandemic, and the pace of vaccination slowed.
BioNTech and Pfizer developed an omicron-adapted booster, but uptake hasn’t matched the original shot. Meanwhile, China, the one big remaining market, continues to rely on locally-designed vaccines even as the end of its Covid Zero policy leads to a surge in infections.
The revenue expectations may be challenging for BioNTech to meet, according to Fazeli. Sales could depend on how much BioNTech and Pfizer can charge for their vaccine as payment shifts from government to insurers in the key US market, he said.
The company itself has been reluctant to make predictions. Because vaccine sales will depend on whether another variant emerges and changes the dynamics of the pandemic, Sahin said, “we can’t just predict what is going to happen.”
©2023 Bloomberg L.P.